Nutrient Supplying Power of Soil
In the past 150 years, CO2 levels in the atmosphere have increased from 260 to
365 ppm (Follett and McConkey, 2000) and it is expected to rise 1.5 to 2.0 ppm
per year (Wittwer, 1985). This increase
is believed to have increased the average temperature of the earth by 0.5 °C
and thus various reports of global warming as a result of increased evolution
of CO2 into earth's atmosphere
(Perry, 1983). It is possible to
decrease the release of CO2 to the
atmosphere by choosing an alternative energy source. However, total control of the release of CO2 is not easy because there are so many
different sources, including the production of cement, gasoline-driven
automobiles, burning of fuels for home heating, cooking, etc. (Wallace et al.,
1990). There are, however, several
benefits associated with increased atmospheric CO2 including
increased water use efficiency, nitrogen use efficiency and production in many
crops.
If
the expected fossil fuel CO2 released for many years could be stored as soil
organic matter, vastly enhanced productive soil would result. This option requires increased biomass to
produce the needed soil organic matter, but this could be achievable due to
increased CO2 supplies in the
atmosphere (Wallace et al., 1990).
Obstacles to increasing the level of soil organic matter are; 1) needed
organic matter supplies, 2) needed nitrogen to give around a 10:1 carbon:nitrogen
ratio necessary for stable soil organic matter, and 3) efficiency in microbial
activity that can result in more stable soil organic matter, instead of burn
out resulting in return of CO2 to the atmosphere (Wallace et al., 1990).
It
is seldom understood that organic matter contents in soils can be increased via
various management practices. Increased
use of no-till management practices can increase soil organic matter. After ten years of no-tillage with corn, soil
organic carbon in the surface 30 cm was increased by 0.25% (Blevins et al.
1983). Probably the least understood is
increased N rates in continuous crop production on resultant soil organic
matter levels. Various authors have
documented that N rates in excess of that required for maximum yields result in
increased biomass production (decreased harvest index values e.g., unit grain
produced per unit dry matter). This
results in increased amounts of carbon from corn stalks, wheat stems, etc.,
that are incorporated back into soil organic matter pools. Although this effect is well documented, the
deleterious effects of increased fertilizer N rates on potential NO3 leaching and/or NO3 surface runoff should be considered where
appropriate. Use of green manures and
animal wastes have obvious impacts on soil organic matter when used on a
frequent basis.
The
native fertility of forest and grassland soils in

Figure
1.1. Influence of cultivation time on
relative mineralization from soil humus and wheat residue. (From Campbell et
al. (1976)).
When
the reddish prairie soils of
N
removal in the Check (no fertilization)
plot of the Magruder Plots
20 bu/acre * 60 lb/bu * 100 years =
120000lbs
120000 lbs * 2%N in the grain = 2400 lbs
N/acre over 100 years
8000 lbs N in the soil (1892)
2000 lbs N in the soil (1992)
2400 lbs N removed in the grain
=3600 lbs N unaccounted
The
effects that management systems will have on soil organic matter and the
resultant nutrient supplying power of the organic pools are well known. Various management variables and their effect
on soil organic matter are listed;
Organic Matter Management Effect
_________________________________________________________
1) tillage +/- conventional -
zero +
2) soil drainage +/-
3) crop residue placement +/-
4) burning -
5) use of green manures +
6) animal wastes and composts +
7) nutrient management +/- excess N +
_________________________________________________________
The
living component which includes soil microorganisms and fauna make up a
relatively small portion of total soil organic matter (1-8%). It functions however as an important catalyst
for transformations of N and other nutrients (Doran and Smith, 1987). The majority of soil organic matter is
contained in the nonliving component that includes plant, animal and microbial
debris and soil humus.
Common
components of soil organic matter and their relative rates of decay are listed
in Table 1.1. Cellulose generally
accounts for the largest proportion of fresh organic material. It generally decays rather rapidly, however,
the presence of N is needed in order for this to take place. Lignin components decompose much more slowly
and thus, any nutrients bound in lignin forms will not become available for
plant growth. Although lignin is
insoluble in hot water and neutral organic solvents, it can be solubilized in
alkali solutions. Because of this, we
seldom find calcareous soils with extremely high organic matter. All of the polysaccharides decompose rapidly
in soils and thus serve as an immediate source of C for microorganisms. Decomposition of these respective components
is illustrated in Figure 1.2.
Table 1.1. Components of soil organic matter, rate of
decomposition and composition of each fraction.
____________________________________________________________________________________
Form Formula Decomposition Composition
____________________________________________________________________________________
Cellulose (C6H10O5)n rapid * 15-50%
Hemicellulose 5-35%
glucose C6H12O6 moderate-slow
galactose
mannose
xylose C5H10O5 moderate-slow

Lignin(phenyl-propane) slow 15-35%
Crude Protein RCHNH2COOH** rapid 1-10%
Polysaccharides
Chitin (C6H9O4.NHCOCH3)n rapid
Starch glucose chain rapid
Pectins galacturonic acid rapid
Inulin fructose units
____________________________________________________________________________________
* - decomposition more rapid
in the presence of N
** - amino
acid glycine (one of many building blocks for proteins)

Figure 1.2. Decomposition of Miscanthus sinensis leaf litter.
Table 1.2. Composition of mature cornstalks (Zea mays L.) initially and after 205
days of incubation with a mixed soil microflora, in the presence and absence of
added nutrients (Tenney and Waksman, 1929)
____________________________________________________________________________________
Initial Composition after 205 days (%)
composition No nutrients Nutrients
Constituents or fraction % added added
____________________________________________________________________________________
Ether and alcohol soluble 6 1 <1
Cold water soluble 11 3 4
Hot water soluble 4 4 5
Hemicelluloses 18 15 11
Cellulose 30 13 6
Lignins 11 23 24
Crude protein 2 9 11
Ash 7 19 26
____________________________________________________________________________________
The
composition of mature cornstalks before and after 205 days of incubation with a
mixed soil in the presence and absence of added nutrients is listed in Table
1.2. As decomposition proceeds, the
water soluble fraction (sugars, starch, organic acids, pectins and tannins and
array of nitrogen compounds) is readily utilized by the microflora (Parr and
Papendick, 1978). Ether and
alcohol-soluble fractions (fats, waxes, resins, oils), hemicelluloses and
cellulose decrease with time as they are utilized as carbon and energy
sources. Lignin, tends to persist and
accumulate in the decaying biomass because of its resistance to microbial
decomposition. Decomposition rates of
crop residues are often proportional to their lignin content and some
researchers have suggested that the lignin content may be a more reliable
parameter for predicting residue decomposition rates than the C:N ratio
(Alexander, 1977). Vigil and Kissel (1991)
included the lignin-to-N ratio and total soil N concentration (in g/kg) as
independent variables to predict potential N mineralization in soil. They also noted that the break point between
net N mineralization and net immobilization was calculated to be at a C/N ratio
of 40.

A
simple illustration of the carbon cycle is found in Figure 1.3. The carbon cycle revolves around CO2, its fixation and regeneration. Chlorophyll-containing plants utilize the gas
as their sole carbon source and the carbonaceous matter synthesized serves to
supply the animal world with preformed organic carbon. Upon the death of the plant or animal,
microbial metabolism assumes the dominant role in the cyclic sequence
(Alexander, 1977). Without the microbial
pool, more carbon would be fixed than is released, CO2 concentrations in the atmosphere would
decrease and photosynthesis rates would decrease.
Figure 1.3. Simple illustration of the carbon cycle (from Alexander, 1977). "Higher plants use light to convert water (H2O) and carbon dioxide (CO2) to glucose (C6H12O6) and oxygen (O2)."
C:N Ratios as Related to Organic Matter Decomposition
In
general, the following C:N ratios are considered to be a general rule of thumb
in terms of what is expected for immobilization and mineralization.
_____________________________________________________
C:N Ratio Effect
_____________________________________________________
30:1 immobilization
<20:1 mineralization
20-30:1 immobilization = mineralization
_____________________________________________________
Unfortunately,
C:N ratios say nothing about the availability of carbon or nitrogen to
microorganisms. The reason for this is
because we are not aware of what makes up the carbon (C) component. In tropical soils, significantly higher
proportions of lignin will be present in the organic matter. Even though the percent N within the organic
matter may be the same, it would be present in highly stable forms that were
resistant to decomposition. Therefore,
mineralization rates in organic matter that contain high proportions of lignin
will be much smaller. The C:N ratios
discussed were generally developed from data obtained in temperate
climates. Therefore their applicability
to tropical soils is at best minimal.
Decomposition of Organic Matter (Mineralization)
1. percent organic matter
2. organic matter composition
3. cultivation (crop, tillage, burning)
4. climate (moisture, temperature)
5. soil pH
6. N management (fertilization)
7. soil aeration
During
the initial stages of decomposition of fresh organic material there is a rapid
increase in the number of heterotrophic organisms accompanied by the evolution
of large amounts of carbon dioxide. If
the C:N ratio of the fresh material is wide, there will be a net N
immobilization. As decay proceeds, the
C:N ratio narrows and the energy supply of carbon diminishes.
The
addition of materials that contain more than 1.5 to 1.7% N would ordinarily
need no supplemental fertilizer N or soil N to meet the demands of the
microorganisms during decomposition (Parr and Papendick, 1978). Note that the 'demands of the microorganisms'
is what is discussed first, with no regard as to what plant N needs might
be. The addition of large amounts of
oxidizable carbon from residues with less than 1.5% N creates a microbiological
demand for N which can immobilize residue N and available inorganic soil N for
extended periods. The addition of
supplemental inorganic fertilizer N to low N residues can accelerate their rate
of decomposition (Parr and Papendick, 1978).
In
the thousands of years prior to the time cultivation was initiated, C and N had
built up in native prairie soils.
However, the C:N ratio was wide, reflecting conditions for
immobilization of N. The combined influence
of tillage and the application of additional organic materials (easily
decomposable wheat straw and/or corn stalks) is illustrated in Figure 1.4. Cultivation alone unleashed a radical
decomposition of the 4% organic matter in

Figure 1.4.
Effect of cultivation and addition of straw materials on immobilization
and mineralization of N and associated evolution of CO2

Figure
1.5. Changes in the nitrogen content of
decomposing barley straw (From Alexander, 1977).

Figure 1.6. Changes in soil mineral N as a function of time, and addition of manure and straw.
Table 1.2. Example calculations
of total N in organic matter fractions in soils and expected amounts of N
mineralized on a yearly basis.
____________________________________________________________________________________
min max min max
____________________________________________________________________________________
Organic Matter, % 1 2 4 12
1 ha (0-15cm), kg 2241653 2241653 2241653 2241653 (Pb = 1.47)
Organic, matter, kg 22416 44833 89666 268998
%
N in
(5%)
kg N in
% N mineralized/yr 0.03 0.03 0.03 0.03
(3%)
TOTAL (kg N/ha/yr) 33.6 67.2 134.4 ? 403.5 ?
____________________________________________________________________________________
DB= Mass of dry soil/volume of solids and voids
2000000 pounds/afs
ft3*0.02832 = m3
0.4535 lb/kg
1 ha = 2.471ac
1 ha = 10000m2
1 ac = 4047m2
2000000 lb = 907184.74 kg = 907.184 Mg
43560 ft2 * 0.5 ft = 21780 ft3 = 616.80m3
907.184Mg/616.80m3 = DB 1.4707
10000m2 * 0.15m = 1500 m3
2241653 kg /1000 = 2241.6 Mg
2241.6/1500 = DB 1.49 (g/cm3 = Mg/m3)
What will happen if
a) bulk density is changed?
b) % N in organic matter?
c) % N mineralized per year?
Organic Matter = 0.35 + 1.80 * (organic carbon) Ranney (1969)
The
most important function of the microbial flora is usually considered to be the
breakdown of organic materials, a process by which the limited supply of CO2 available for photosynthesis is replenished
(Alexander, 1977).
Five
major groups of microorganisms in the soil are:
1. Bacteria
2. Actinomycetes
3. Fungi
4. Algae
5. Protozoa
Soil
Bacteria: 108 to 1010 / g of soil
Heterotroph:
(chemoorganotrophic) require preformed organic nutrients to serve as sources of
energy and carbon.
1. Fungi
2. Protozoa
3. Most Bacteria
Autotroph:
(lithotrophic) obtain their energy from sunlight or by the oxidation of inorganic
compounds and their carbon by the assimilation
of CO2.
Photoautotroph:
energy derived from sunlight
1. Algae (blue-green, cyanobacteria)
2. Higher Plants
3. Some Bacteria
Chemoautotroph:
energy for growth obtained by the oxidation of inorganic materials.
1. Few Bacterial species (agronomic
importance)
a. nitrobacter,
nitrosomonas and thiobacillus
Arnon's Criteria of Essentiality
1.
Element required
to complete life cycle.
2.
Deficiency can
only be corrected by the ion in question.
3.
Element needs to
be directly involved in the nutrition of the plant and not indirectly via the
need of another organism.
Any mineral element that functions in plant
metabolism, whether or not its action is specific (Tisdale et al., 1985).
C, H, O, N, P, and S (constituent of proteins)
Ca, Mg, K, Fe, Mn, Mo, Cu, B, Zn, Cl, Na, Co, V, Si
(essential to one or more plants)
'CHOPKNS CaFe MgB Mn Cl CuZn Mo'
Mobile Nutrients
A. Plant
1.
deficiency symptoms appear in the lower older leaves
B. Soil
1. can
be taken up from a large volume of soil
Immobile Nutrients
A. Plant
1. deficiency
symptoms appear in the upper younger leaves
B. Soil
1.
taken up from a small volume of soil
Deficiency Symptom Element Mobility Mobility Form taken up
Soil Plant by Plants
____________________________________________________________________________________
overall
chlorosis seen N Nitrogen Yes Yes NO3-,NO2-,NH4+
first on
lower leaves
purple
leaf margins P
Phosphorus No Yes HPO4=,H2PO4-,H3PO4
chlorotic
leaf margins K Potassium No Yes K+
uniform
chlorosis, stunting
(younger
leaves) S Sulfur Yes Yes(no) SO4=,SO2*
N*S
interaction
stunting -
no root
elongation Ca Calcium No No Ca++
interveinal
chlorosis,
veins
remain green Fe Iron No (ls) No Fe+++,Fe++
interveinal
chlorosis Mg Magnesium No (ls) Yes/No Mg++
reduced
terminal
growth =
chlorotic tips B Boron (NM) Yes No H3BO3°
interveinal
chlorosis Mn Manganese No No Mn++, Mn+++
wilting,
chlorosis, reduced
root
growth Cl
Chlorine Yes Yes Cl -
young
leaves, yellow &
stunted Cu Copper No (ls) No Cu++
interveinal
chlorosis in
young
leaves Zn Zinc No (ls) No Zn++
interveinal
chlorosis,
stunting Mo
Molybdenum Yes/No(ls) No MoO4=
dark green
color Na Sodium No(ls) Yes Na+
C Carbon CO2
H Hydrogen H2O
O Oxygen H2O
____________________________________________________________________________________
* absorbed
through plant leaves
(NM) Non
Metal
(ls) Low
Solubility
Mo
availability increases with soil pH, other micronutrients show the opposite of
this.
Immobile nutrients
in plant; symptoms of deficiency show up in the younger leaves.
Stage of
growth when deficiency symptom is apparent = later stage
NITROGEN:
·
Key building
block of the protein molecule upon which all life is based
·
Indispensable
component of the protoplasm of plants animals and microorganisms
·
One of the few
soil nutrients lost by volatilization and leaching, thus requiring continued
conservation and maintenance
·
Most frequently
deficient nutrient in crop production
Nitrogen Ion/Molecule Oxidation States
Nitrogen ions and molecules that
are of interest in soil fertility and plant nutrition cover a range of N
apparent oxidation states from -3 to +5.
It is most convenient to illustrate these oxidation states using common
combinations of N with H and O, because H can be assumed in the +1 oxidation
state (H+1) and O in the -2 oxidation state (O=). The apparent N oxidation state, and the
electron configurations involved may be depicted as follows.
Hydrogen:
The electron configuration in the
ground state is 1s1 (the first electron shell has only one electron in it), as
found in H2 gas. Since the s
shell can hold only two electrons, the atom would be most stable by either
gaining another electron or losing the existing one. Gaining an electron by sharing occurs in H2,
where each H atom gains an electron from the other resulting in a pair of
electrons being shared. The electron
configuration about the atom, where: represent a pair of electrons, may be
shown as
H:H
and the bond may be shown as H-H
Hydrogen most commonly exists in
ionic form and in combination with other elements where it has lost its single
electron. Thus it is present as the H+
ion or brings a + charge to the molecule formed by combining with other elements.
Oxygen:
The ground state of O, having a
total of eight electrons is 1s2, 2s2, 2p4.
Both s orbitals are filled, each with two electrons. The 2p outer or valence orbital capable of
holding six electrons, has only four electrons, leaving opportunity to gain
two. The common gain of two electrons
from some other element results in a valence of -2 for O (O=). The gain of two electrons also occurs in O2
gas, where two pairs of electrons are shared as
O::O
and the double bond may be shown as O=O
Nitrogen:
The ground state of N is 1s2, 2s2,
2p3. It is very similar to that for
oxygen, except there is one less electron in the valence 2p orbital. Hence, the 2p orbital contains three
electrons but, has room to accept three electrons to fill the shell. Under normal conditions, electron loss to
form N+, N2+ or N3+ or electron gain to form N-,
N2-, or N3- should not be expected. Instead, N will normally fill its 2p orbital
by sharing electrons with other elements to which it is chemically (covalent)
bound. Nitrogen can fill the 2p orbital
by forming three covalent bonds with itself as in the very stable gas N2.
The Nitrogen cycle is not well
understood, largely because of how it is communicated. Similar to the way we communicate the
differences between normal, saline, sodic and saline-sodic soils, we should do
the same for response variables in the Nitrogen cycle. In addition to temperature and pH included
below, we could add reduction/oxidation, tillage (zero vs. conventional), C:N
ratios, fertilizer source and a number of other variables. These mechanistic models would ultimately
lead to many 'if-then' statements/decisions that could be used within a
management strategy.
>50F
|
denitrification |
volatilization |
|
leaching |
leaching |
<50F 7.0
Soil
pH
Assuming that we could speed up
the nitrogen cycle what would you change?
1.
Aerated environment (need for O2)
2.
Supply of ammonium
3.
Moisture
4.
Temperature (30-35C or 86-95F) <10C or 50F
5.
Soil pH
6.
Addition of low C:N ratio materials (low lignin)
Is oxygen required for
nitrification?
Does nitrification proceed during
the growing cycle? (low C:N ratio)
N Oxidation States:
oxidized: loses electrons, takes
on a positive charge
reduced: gains electrons, takes on
a negative charge
![]() Ion/molecule Name
Ion/molecule Name
NH3 ammonia -3

NH4+ ammonium -3
![]()
N2 diatomic
N 0
![]()
N2O nitrous
oxide +1
![]()
NO nitric oxide +2
-
![]()
+ -
NO2- nitrite +3

-
NO3- nitrate +5
_____________________________________
H2S hydrogen
sulfide -2
SO4= sulfate +6
_____________________________________
N:
5 electrons in the outer shell
·
loses 5 electrons
(+5 oxidation state NO3)
·
gains 3 electrons
(-3 oxidation state NH3)
O: 6 electrons in the outer shell
·
is always being
reduced (gains 2 electrons to fill the outer shell)
H:
1 electron in the outer shell
N
is losing electrons to O because O is more electronegative
N
gains electrons from H because H wants to give up electrons
_______________________________________________
N recommendations
1.
Yield goal (2lb N/bu)
a. Applies fertilization risk on the
farmer
b. Removes our inability to predict
'environment' (rainfall)
2.
Soil test
a. For every 1 ppm NO3, N recommendation reduced by 2lbN/ac
Nitrite accumulation?
1.
high pH
2.
high NH4 levels (NH4 inhibits nitrobacter)

NITROGEN
CYCLE
Inorganic
nitrogen buffering is defined as the ability of the soil-plant system to
control the amount of inorganic N accumulation in the rooting profile when N
fertilization rates exceed that required for maximum yield.
If
N rates required to detect soil profile NO3 accumulation
always exceeded that required for maximum yields, what biological mechanisms
are present that cause excess N applied to be lost via other pathways prior to
leaching?

Nitrogen Buffering Mechanisms
1. Increased Applied N results in increased plant N
loss (NH3)
2. Higher rates of applied N - increased
volatilization losses
3. Higher rates of applied N - increased
denitrification
4. Higher rates of applied N - increased organic C, --
increased N in organic pools
5. Increased applied N - increased grain protein
6. Increased applied N - increased forage N
7. Increased applied N - increased straw N
· Urease
activity ·
Air Exchange
·
Temperature ·
N Source and Rate
· CEC
(less when high) ·
Application method
· H
buffering capacity of the soil ·
Crop Residues
· Soil
Water Content
NH4+ « NH3 + H+
If pH and temperature can be kept
low, little potential exists for NH3
volatilization. At pH 7.5, less than 7%
of the ammoniacal N is actually in the form of NH3 over the range of temperatures likely for
field conditions.
A+B « AB
Kf = AB/A x B
AB « A+B
Kd =
A x B/AB
Kf = 1/Kd (relationship between formation and dissociation
constants)
Formation constant (Log K°)
relating two species is numerically equal to the pH at which the reacting
species have equal activities (dilute solutions).
pKa and Log K° are sometimes synonymous
Henderson-Hasselbalch
pH = pKa + log [(base)/(acid)]
when (base) = (acid), pH = pKa
· Urea is the most important solid fertilizer in the world today.
· In the early 1960's, ammonium sulfate was the primary N product in world trade (Bock and Kissel, 1988).
·
The majority of all urea production in the
· Since 1968, direct application of anhydrous ammonia has ranged from 37 to 40% of total N use (Bock and Kissel, 1988).
· Urea: high analysis, safety, economy of production, transport and distribution make it a leader in world N trade.
· In 1978, developed countries accounted for 44% of the world N market (Bock and Kissel, 1988).
· By 1987, developed countries accounted for less than 33%.
Share of world N consumption by product group
1970 1986
Ammonium nitrate 27 15
Urea 9 37
Ammonium phosphates 1 5
Other N products (NH3) 36 29
Other complex N
products 16 8
increase pH (less H+ ions in soil solution)
CO(NH2)2 + H+ + 2H2O --------> 2NH4+ + HCO3-
pH 6.5 to 8
HCO3- + H+ --->
CO2 + H2O
(added H lost from soil solution)
CO(NH2)2 + 2H+ + 2H2O --------> 2NH4+ + H2CO3 (carbonic acid)
pH <6.3

Potential for gaseous loss from applied urea, both broadcast and incorporated.
During hydrolysis, soil pH can
increase to >7 because the reaction requires H+ from the soil
system.
(How many moles of H+ are consumed for each mole of urea hydrolyzed?) 2
In alkaline soils less H+ is initially needed to drive urea hydrolysis on a
soil already having low H+.
In an alkaline soil, removing more
H+(from a soil solution already low in H+),
can increase pH even higher
NH4+ + OH- ---> NH4OH ---->NH3 + H2O
pH = pKa + log [(base)/(acid)]
At a pH of 9.3 (pKa 9.3) 50% NH4
and 50% NH3
pH Base (NH3) Acid (NH4)
7.3 1 99
8.3 10 90
9.3 50 50
10.3 90 10
11.3 99 1

Equilibrium relationship for ammoniacal N and resultant amount of NH3 and NH4 as affected by pH for a dilute solution.
As the pH increases from urea
hydrolysis, negative charges become available for NH4+ adsorption because of the release of H+ (Koelliker and Kissel)
Decrease NH3 loss with increasing CEC (Fenn and Kissel, 1976)
assuming increase pH = increase
CEC, what is happening?
In acid soils, the exchange of NH4+ is for H+ on the exchange complex (release of H here,
resists change in pH, e.g. going up)
In alkaline soils with high CEC, NH4 exchanges for Ca,
precipitation of CaCO3 (CO3= from HCO3- above) and one H+ released which helps resist the increase in pH
However, pH was already high.

Soil surface pH and cumulative NH3 loss as influenced by pH buffering capacity (from Ferguson et al., 1984).
Ernst and Massey (1960) found
increased NH3 volatilization when liming a silt loam soil. The effective CEC would have been increased
by liming but the rise in soil pH decreased the soils ability to supply H+
Rapid urea hydrolysis: greater potential for NH3 loss. Why?
management: dry soil surface,
incorporate, localized placement- slows urea hydrolysis
H
ion buffering capacity of the soil:
Ferguson et al., 1984
(soils total acidity, comprised of
exchangeable acidity + nonexchangeable titratable acidity)
A large component of a soils total
acidity is that associated with the layer silicate sesquioxide complex (Al and
Fe hydrous oxides). These sesquioxides
carry a net positive charge and can hydrolyze to form H+ which resist an increase in pH upon an addition of a
base.
H+ ion supply
comes from:
1.
2.
hydrolysis of water
3.
Al and Fe hydrous oxides
4.
high clay content
A soil with an increased H+ buffering capacity will also show less NH3 loss when urea is applied without
incorporation.
1. hydroxy Al-polymers added (carrying a net positive
charge) to increase H+ buffering capacity.
2. strong acid cation exchange resins added (buffering
capacity changed without affecting CEC, e.g. resin was saturated with H+).
resin: amorphous organic substances (plant
secretions), soluble in organic solvents but not in water (used in plastics,
inks)
Consider the following
1. H+ is required for urea hydrolysis.
2. Ability of a
soil to supply H+ is related to amount of NH3 loss.
3. H+ is produced via nitrification (after urea is
applied): acidity generated is not beneficial.
4. What could
we apply with the urea to reduce NH3 loss?
an acid; strong electrolyte;
dissociates to produce H+;increased H+ buffering; decrease pH
reduce NH3 loss by maintaining a low pH in the vicinity of the
fertilizer granule (e.g. H3PO4)
Factors Affecting Soil Acidity
Acid: substance
that tends to give up protons (H+) to some other
substance
Base: accepts
protons
Anion: negatively
charged ion
Cation: positively
charged ion
Base cation: ? (this has been taught in the past but
is not correct)
Electrolyte: nonmetallic electric conductor in which
current is carried by the movement of ions
H2SO4 (strong electrolyte)
CH3COOH (weak electrolyte)
H2O
HA --------------> H+ + A-
potential active
acidity acidity
1. Nitrogen
Fertilization
A.
ammoniacal sources of N
2. Decomposition
of organic matter
CO2 + H2O --------> H2CO3 (carbonic acid)
H2CO3 ------> H+ + HCO3-
(bicarbonate)
humus
contains reactive carboxylic, phenolic groups that behave as weak acids which
dissociate and release H+
3. Leaching of
exchangeable bases/Removal
Ca, Mg, K
and Na (out of the effective root zone)
-problem in
sandy soils with low CEC
a. Replaced first by H and subsequently by Al (Al
is one of the most abundant elements in soils.
7.1% by weight of earth's crust)
b. Al displaced from clay minerals, hydrolyzed to
hydroxy aluminum complexes
c. Hydrolysis of monomeric forms liberate H+
d. Al(H2O)6+3 + H2O ----->
Al(OH)(H2O)++ + H2O+
monomeric: a
chemical compound that can undergo polymerization
polymerization:
a chemical reaction in which two or more small
molecules combine to form larger molecules that contain repeating
structural units of the original molecules
4. Aluminosilicate
clays
Presence of
exchangeable Al
Al+3
+ H2O -----> AlOH= + H+
5. Acid Rain
Acidification from N Fertilizers (R.L. Westerman)
1. Assume that
the absorbing complex of the soil can be represented by CaX
2. Ca represents
various exchangeable bases with which the insoluble anions X are combined in an
exchangeable form and that X can only combine with one Ca
3. H2X refers to dibasic acid (e.g., H2SO4)
(NH4)2SO4 -----> NH4+ to the exchange complex, SO4= combines with the base on the exchange complex
replaced by NH4+
Thought: Volatilization losses of N as NH3 preclude the development of H+ ions produced via nitrification and would
theoretically reduce the total potential development of acidity.
Losses
of N via denitrification leave an alkaline residue (
Table X. Reaction of N
fertilizers when applied to soil.
______________________________________________________________________
1. Ammonium sulfate
a. (NH4)2SO4 + CaX
----> CaSO4 + (NH4)2X
b. (NH4)2X + 4O2 nitrification
>2HNO3 + H2X + 2H2O
c. 2HNO3 + CaX
----> Ca(NO3)2 + H2X
Resultant
acidity = 4H+ /mole of (NH4)2SO4
2. Ammonium nitrate
a. 2NH4NO3 + CaX ----> Ca(NO3)2 + (NH4)2X
b. (NH4)2X + 4O2 nitrification
>2HNO3 + H2X + 2H2O
c. 2HNO3 + CaX
----> Ca(NO3)2 + H2X
Resultant
acidity = 2H+ /mole of NH4NO3
3. Urea
a. CO(NH2)2 + 2H2O ---->
(NH4)2CO3
b. (NH4)2CO3 + CaX ---->
(NH4)2X + CaCO3
c. (NH4)2X + 4O2 nitrification
>2HNO3 + H2X +2H2O
d. 2HNO3 +CaX
----> Ca(NO3)2 + H2X
e. H2X + CaCO3 neutralization >CaX + H2O + CO2
Resultant
acidity = 2H+ /mole of CO(NH2)2
4. Anhydrous Ammonia
a. 2NH3 +2H2O ----> 2NH4OH
b. 2NH4OH + CaX
----> Ca(OH)2 + (NH4)2X
c. (NH4)2X + 4O2 nitrification >2HNO3 + H2X +2H2O
d. 2HNO3 + CaX
----> Ca(NO3)2 + H2X
e. H2X +
Ca(OH)2 neutralization
> CaX + 2H2O
Resultant
acidity = 1H+/mole of NH3
5. Aqua Ammonia
a. 2NH4ON +
CaX ----> Ca(OH)2 + (NH4)2X
b. (NH4)2X + 4O2 nitrification >2HNO3 + H2X +2H2O
c. 2HNO3 +CaX
----> Ca(NO3)2 + H2X
d. H2X +
Ca(OH)2 neutralization
> CaX +2H2O
Resultant
acidity = 1H+/mole of NH4OH
6. Ammonium Phosphate
a. 2NH4H2PO4 + CaX
----> Ca(H2PO4)2 + (NH4)2X
b. (NH4)2X + 4O2 nitrification
>2HNO3 + H2X +2H2O
c. 2HNO3 +CaX
----> Ca(NO3)2 + H2X
Resultant
acidity = 2H+/mole of NH4H2PO4
______________________________________________________________________
In
grain production systems, N use efficiency seldom exceeds 50 percent. Variables which influence N use efficiency
include
a. Variety
b. N
source
c. N
application method
d. Time
of N application
e. Tillage
f. N
rate (generally decreases with increasing N applied)
g. Production
system
1. Forage
2. Grain
Olson
and Swallow, 1984 (27-33% of the applied N fertilizer was removed by the grain
following 5 years)
h. Plant
N loss
i. Soil
type (organic matter)
Calculating N Use Efficiency using The Difference Method
______________________________________________________________________
Applied N Grain
Yield N content N uptake Fertilizer Recovery
kg/ha kg/ha % kg/ha %
______________________________________________________________________
0 1000 2.0 20 -
50 1300 2.1 27.3 (27.3-20)/50=14.6
100 2000 2.2 44 (44-20)/100=24
150 2000 2.3 46 (46-20)/150=17
______________________________________________________________________
Estimated N use efficiency for grain production
systems ranges between 20 and 50%. The
example above does not include straw, therefore, recovery levels are
lower. However, further analysis of forage
production systems (Altom et al., 1996) demonstrates that N use efficiency can
be as high as 60-70%. This is largely
because the plant is harvested prior to flowering thus minimizing the potential
for plant N loss. Plant N loss is known
to be greater when the plant is at flowering and approaching maturity. It is important to observe that estimated N
use efficiencies in forage production systems do not decrease with increasing N
applied as is normally found in grain production systems. This is suggestive of 'buffering' whereby
increased N is lost at higher rates of applied N in grain production systems,
but which cannot take place in forage production systems.
Work by Moll et al. (1982) suggested the presence of
two primary components of N use efficiency: (1) the efficiency of absorption or
uptake (Nt/Ns), and (2) the efficiency with which the N absorbed is utilized to
produce grain (Gw/Nt) where Nt is the total N in the plant at maturity (grain +
stover), Ns is the nitrogen supply or rate of fertilizer N and Gw is the grain
weight, all expressed in the same units.
Other parameters defined in their work and modifications (in italics)
are reported in Table 4.2.

Recent
understanding of plant N loss has required consideration of additional
parameters not discussed in Moll et al. (1982).
Harper et al. (1987) documented that N was lost as volatile NH3
from wheat plants after fertilizer application and during flowering. Maximum N accumulation has been found to
occur at or near flowering in wheat and corn and not at harvest. In order to estimate plant N loss without the
use of labeled N forms, the stage of growth where maximum N accumulation is
known to occur needs to be identified.
The amount of N remaining in the grain + straw or stover, is subtracted
from the amount at maximum N accumulation to estimate potential plant N loss
(difference method). However, even the
use of difference methods for estimating plant N loss are flawed since
continued uptake is known to take place beyond flowering or the point of
maximum N accumulation.
Figure 4.1 Total N uptake in winter wheat with time and estimated loss following flowering.
Francis et al. (1993) recently documented that plant N
losses could account for as much as 73% of the unaccounted-for N in 15N
balance calculations. They further noted
that gaseous plant N losses could be greater when N supply was increased. Similar to work by Kanampiu et al. (1997)
with winter wheat, Francis et al. (1993) found that maximum N accumulation in
corn occurred soon after flowering (R3 stage of growth). In addition, Francis et al. (1993)
highlighted the importance of plant N loss on the development and
interpretations of strategies to improve N fertilizer use efficiencies.
Consistent with work by Kanampiu et al. (1997), and
Daigger et al. (1976), Figure 4.1 illustrates winter wheat N accumulation over
time. Estimates of plant N loss are
reported in Table 4.1. Harper et al. (1987) reported that 21% of the applied N
fertilizer was lost as volatile NH3 in wheat, of which 11.4% was
from both the soil and plants soon after fertilization and 9.8% from the leaves
of wheat between anthesis and physiological maturity. Francis et al. (1993) summarized that failure
to include direct plant N losses when calculating an N budget leads to
overestimation of N loss from the soil by denitrification, leaching and ammonia
volatilization.
![]()
![]()
NO3- + 2e (nitrate reductase) NO2-
+ 6e (nitrite reductase) NH4+
Reduction of NO3- to NO2-
is the rate-limiting step in the transformation of N into amino forms.
Does the plant wake up in the morning and turn on the
TV to check the weather forecast, to see if it should assimilate NO3
and attempt to form amino acids?
Could we look at the forecast and attempt to
communicate with the plant, letting it know that weather conditions will be
good (or bad), thus proceeding with increased NO3 uptake?
Major pathways for assimilation of NH3
1. Incorporation
into glutamic acid to form glutamine, a reaction catalyzed by glutamine synthetase
(Olson and Kurtz, 1982)
2. Reaction of
NH3 and CO2 to form carbamyl phosphate, which in turn is
converted to the amino acid arginine.
3. Biosynthesis
of amides by combination of NH3 with an amino acid. In this way aspartic acid is converted to the
amide, asparagine.
Table 4.1. Means over N rate and variety for
protein, NUE components and estimated plant N loss,
_______________________________________________________________________________________________
Protein N-use Uptake N-utilization Fraction of Grain
yield/ N loss
% efficiency efficiency efficiency N
translocated grain N (kg ha-1)
(Gw/Ns) (Nt/ Ns) (Gw/Nt) to grain(Ng/Nt) (Gw/Ng) (
N rate,
kg ha-1 --------------------------------------------------------
means --------------------------------------------------------
0 14.8 0 0 23.2 0.60 38.8 16.4
45 15.9 23.3 1.0 22.9 0.63 36.5 25.0
90 17.4 11.0 0.6 20.2 0.61 33.2 25.8
180 17.6 7.0 0.4 20.5 0.62 33.5 31.4
SED 0.40 1.1 0.05 1.12 0.03 0.89 6.74
Variety:
Chisholm 16.3 11.8 0.5 22.4 0.6 35.3 21.8a
Karl 17.5 13.1 0.6 23.0 0.7 33.0 26.6a
2180 17.4 18.1 0.8 22.7 0.7 33.4 27.9a
TAM
W-101 15.5 11.7 0.6 21.4 0.6 37.4 24.7a
Longhorn 15.0 14.7 0.8 19.5 0.5 38.5 22.3a
SED 0.45 1.5 0.07 1.27 0.04 1.18 7.33
_______________________________________________________________________________________________
The ability of the soil-plant system to efficiently
utilize N for food production (grain or forage) can be considered in four
aspects: (1) efficiency of the plant to assimilate applied N, (2a and 2b) once
assimilated, the ability of the plant to retain and incorporate N into the
grain, (3) efficiency of the soil to supply/retain applied N for plant
assimilation over long periods of time and (4) composite system efficiency.
Uptake efficiency should be estimated using Nf/Ns
(Eup) instead of Nt/Ns (Eha) as proposed by Moll et al. (1982). More N is assimilated at earlier stages of
growth, therefore, uptake efficiency should be estimated at the stage of
maximum N accumulation and not at maturity when less N can be accounted
for. The component Nt/Ns as proposed by
Moll et al. would be better defined as harvest uptake efficiency or physiological
maturity uptake efficiency. We define
uptake efficiency as the stage where maximum N is taken up by the plant divided
by the N supplied.
(1)
Uptake efficiency Eup=Nf/Ns
Unlike the description by Moll et al. (1982), uptake
efficiency should be partitioned into two separate components since plant N
loss (from flowering to maturity) can be significant (Daigger et al., 1976;
Harper et al., 1987; Francis et al., 1993).
The fraction of N translocated to the grain should be estimated as Ng/Nf
and not Ng/Nt as proposed by Moll et al. (1982) since more N was accumulated in
the plant at an earlier stage of growth (Kanampiu et al., 1997). Plants losing significant quantities of N as
NH3 would have very high fractions of N translocated to the grain
when calculated using Nt instead of Nf. In
terms of plant breeding efforts, this could be a highly misleading
statistic. A second component, the
translocation index is proposed that would reflect the ability of a plant
genotype or management practice to incorporate N accumulated at flowering into
the grain.
(2a)
fraction of N translocated to the grain Et=Ng/Nf
(2b)
translocation index Eti=Ng/Nf
* (1/Nl)
The ability of the soil-plant system to utilize
outside sources of N for food production (grain or forage) depends on the
efficiency of storage in the soil. The
efficiency of the soil to supply N to plants is strongly influenced by
immobilization and mineralization with changing climate and environment.
Over a growing season, storage efficiency will be
equal to the difference between fertilizer N added (Ns) minus maximum plant
uptake (Nf) plus the difference between total soil N at the beginning and end
of the season, all divided by fertilizer N added.
Esg =
[(Ns-Nf)-(St1-St2)]/Ns
(3) soil (management system) supply efficiency Es=Ns/(Sv+Sd+Sl) where
Lastly,
a composite estimate of efficiency for the entire system (soil and plant) can
be estimated as follows
(4)
composite system efficiency Ec=Eup*Es=Nf/(Sv+Sd+Sl)
It is important to note that these efficiency
parameters can be determined without having to determine total N in the
soil. Avoiding total soil N analyses is
noteworthy since the precision of present analytical procedures (Kjeldahl or
dry combustion) approach ± 0.01%. This
translates into approximately ± 220 kg N/ha (depending on soil bulk density)
which is often greater than the rate of N applied, thus restricting the ability
to detect N treatment differences.
Will increased NUE lead to increased NO3
leaching?
Data from Kanampiu et al. (1995)
NUE Sinks: Increased
NUE No Change
-------------
kg / ha --------------
Total N Applied 180 180
Plant N uptake (at flowering) 68 71
Final Grain N uptake 42 40
Plant N loss 26 31
Denitrification 10 15
Immobilization 80 80
Balance 22 14
Leaching ? ?
Table 4.2. Components of nitrogen use efficiency as reported by Moll et al. (1982) and modifications (in bold italics) for grain crops.
Component Abbreviation Unit
Grain weight Gw kg ha-1
Nitrogen supply (rate of fertilizer N) Ns kg ha-1
Total N in the plant at maturity (grain + stover) Nt kg ha-1
N accumulation after
silking Na kg ha-1
N accumulated in grain at harvest Ng kg ha-1
Stage of growth where N accumulated in
the plant
is at a maximum, at or near flowering Nf kg ha-1
Total N accumulated in the straw at
harvest Nst kg ha-1
Estimate of gaseous loss of N from the
plant Nl =
Flowering uptake efficiency Eup=Nf/Ns
Harvest uptake efficiency (Uptake efficiency) Eha=Nt/Ns
Translocation index (accumulated N at
flowering
translocated to the grain) Eti
=Ng/Nf * (1/Nl)
Soil supply efficiency Es=Ns/(Sv+Sd+Sl)
Composite system efficiency Ec=Eup*Es=Nf/(Sv+Sd+Sl)
Utilization efficiency Gw/Nt
Efficiency of use Gw/Ns
Grain produced per unit of grain N Gw/Ng
Fraction of total N translocated to grain Et=Ng/Nt
Fraction of total N accumulated after silking Na/Nt
Ratio of N translocated to grain to N accumulated Ng/Na
after silking
Magruder Plots
1892: 4.0 % organic matter = 0.35+ 1.8 OC
OC = 2.03%
TN = 0.16%
Pb = 1.623 (0-12")
lb N/ac = DB * ppm N * 2.7194
= 1.623 * 1600 * 2.7194
=7061
1997
OC = 0.62%
TN = 0.0694%
lb N/ac = 1.623*694*2.7194
=3063
Difference: 7061 - 3063 = 3998
lbs N
Grain N removal
14.6 bu/ac * 60 lb/bu = 876 lbs
876 lbs * 105 years = 91980 lbs grain
91980 lbs * 0.022086 %N = 2031 lbs N
Plant N loss
10.7 lb/ac/yr (Kanampiu et al., 1995)
105 * 10.7 = 1130 lbs N
Denitrification
2.85 lb/ac/yr (Aulakh et al. 1984)
105 * 2.85 = 300 lbs N
Balance 537 lbs N
Year 1 denitrification, ammonification
Denitrification, ug/g = 50.0 * OC + 6.2 (Burford and Bremner, 1975)
= 50.0 * 2.03 + 6.2
= 107.7 ug/g
= 107.7 * 1.623 * 2.7194
= 475.34 lb/ac (0-12")
New Balance 61.66 lb N/ac
(0.58 lb N/ac/yr unaccounted)
Not included in this balance sheet is the amount of N that would be deposited via rainfall, and the amount lost via ammonification, both of which would be important.
Denitrification losses the first year were likely much higher since increased NO3-N would have been present as a result of mineralized N from a very large total N pool. Burford and Bremner (1975) applied the equivalent of 800 lb NO3-N/ac and found that denitrification losses were extremely high. Although their work has little relevance to annual denitrification losses expected under field conditions, it does provide some insight into what might have happened in the first year when soils were first tilled.
Miscellaneous
When adequate inorganic N was present, the incorporation of straw in conventional till or the application of straw on the surface of zero till approximately doubled the accumulative gaseous N losses (increased supply of energy to denitrifying organisms) (Aulakh et al., 1984).
From 71 to 77% of the surface applied fertilizer N remaining in the profiles was in the 0 to 0.1 m soil layers (Olson and Swallow, 1984).
Late N application can be efficiently taken up by plants, and does not decrease soil N uptake. To achieve acceptable grain protein levels for bread wheat in this irrigated cropping system, N should be supplied late in the season to improve N uptake during grain fill (Wuest and Cassman, 1992)
5. Use of Stable and Radioactive Isotopes
Einstein: Relativity theory
(1905), quantum theory
Roentgen: discovered x-rays
Becquerel: first recognition of radioactivity
Bremsstrahlung: identified
secondary x-rays
Curie - Joliot: first induced
artificial radioactivity (1934)
Isotopes are atoms of the same
element that differ in mass. They have the same number of protons and electrons
but have a different mass which is due to the number of neutrons.
1. All radio isotopes have a
particular kind of radiation emission
2. Energy and mass are equivalent
(Einstein)
3. All radio nuclides have a
characteristic energy of radiation
4. All radio nuclides possess a
characteristic rate of decay
1 mole of X has 6.025 x 1023 atoms
one gram of 14N has (14
g/mole)
6.025 x 1023 atoms/mole * 1 mole/14g = 4.3 x 1022 atoms/g
Avogadros # = # of molecules in one
gram molecular weight of any substance.
Dealing with reactions in the
outer ring that compromise and produce chemical reactions.
__________________________________________
atomic mass
units charge
(amu)
__________________________________________
proton 1.007594 +
electron 0.000549 -
neutron 1.008986 none
__________________________________________
mZE 11H 42He
E- element
m - mass
z - atomic number (# of protons in
the nucleus)
All hydrogen atoms have one proton
__________________________________________
11H 21H 31H
__________________________________________
stable stable radioactive
deuterium tritium
mass
= 1 mass=2 mass=3
no
neutron 1 neutron 2 neutrons
1
proton 1 proton 1 proton
1
electron 1 electron 1 electron
__________________________________________
126C 136C 146C
__________________________________________
stable stable radioactive
mass=12 mass=13 mass=14
6
neutrons 7 neutrons 8 neutrons
6
protons 6 protons 6 protons
6
electrons 6 electrons 6 electrons
__________________________________________
Chemical
versus Nuclear Reactions:
1. 2Na+ + H2O ----> 2NaOH + 2H+
3-5
eV in this reaction
2. 42He + 94Be ----> 126C + 10n
10
million eV in this reaction
In a
nuclear reaction, we have to balance both mass and proton number.
Transmutation:
changing one element into another
3517Cl + 10n
------> 3215P + 42He
3216S + 10n
------> 3215P + 11p
Chemical
reactions involve changes in the outer electronic structure of the atom whereas
nuclear reactions involve changes in the nucleus
_____________________________________________________
Radiation
Units/Definitions:
_____________________________________________________
erg: work done by a force of one dyne acting through a distance
of 1 cm.
= 1.0 dyne/cm of 1.0 g - cm2/sec2
dyne: force that would give a free mass of one gram, an
acceleration of one centimeter per second per second
Curie: amount of any radioactive material in which 3.7 x 1010 atoms disintegrate (decay or loss of radioactivity)
per second.
1 Bq (becquerel) 1 dps
1 uC = 3.7 x 104 dps
1 mC = 3.7 x 107 dps = 2.22 x 109 dpm
1 C = 3.7 x 1010 dps = 2.22 x 1012 dpm
Rad = 100 ergs/g absorbing material (quantity of
radiation equivalent to 100 ergs/g of exposed tissue).
1 Rad = 1/100 Roentgen
eV =
electron volt (amount of energy required to raise one electron through a
potential of one volt)
1 eV
= 1.6 x 10-12 erg
1 MeV
= 1.6 x 10-6 erg
specific ionization: # of ion pairs produced/unit distance penetrated.
_____________________________________________________
13755Cs (30
year half life) and 9038Sr (28 year half life) were the
major radioactive isotopes of concern in that accident
Production
Methods:
1.
Particle accelerators
2.
Nuclear reactors
3.
Atomic explosions
Mass
Energy Equivalents:
E = MC2
1 amu
= 1.66 x 10-24 g
=
reciprocal of Avogadro's #
E =
energy (ergs)
M =
mass (grams)
C =
velocity of light (cm/sec)
= 186000 miles/sec
= 3 x 1010 cm/sec
How
much energy does 1 amu have?
E =
(1.66 x 10-24 g) (3 x 1010 cm/sec)2
=1.49 x 10-3 ergs
= (1.49 x 10-3 ergs)/(1.6 x 10-6 erg/Mev) = 931 MeV
Calculate
the amount of energy in 1 gram of 235U?
1g/235g/mole
x 6.025 x 1023 atoms/mole x 0.215amu/atom x 931MeV/amu
=
5.12 x 1023 MeV
= 2.3
x 1014 kilowatt hours
(12 years of electricity for 1 household)
1
kilowatt hour = 2.226 x 109 MeV
only
1/5 or 0.215 of 235U is converted to energy (split)
Fusion: Making hydrogen atoms
combine resulting in released energy
-no remnant radioactivity
-no atmospheric contamination
21H + 31H
---> 42He + 10n
deuterium tritium (alpha)
2½ gallons of tritium would provide the
Fission: "Splitting
atoms"
-results in the production of
radioactive materials
23592U + 10n ---> 9736Kr + 13856Ba +10n + energy
23592U + 10n ---> 9038Sr + 14454Xe + 2 10n + energy
13856Ba is a fission fragment
Strictly chance of actually
knowing what we will have as products from the bombardment of 23592U with neutrons.
23592U "controlled reaction that is a chain
reaction" using uranium rods
238U accounts for 99.3 percent of the uranium found on
earth
23592U is used for fission, because it splits easier.
neutrons emitted in fission can produce a chain
reaction
Nuclear fission taps about 1/1000 of the total
possible energy of the atom.
A. Particulate
1. Alpha (nucleus of the He atom, mass = 4 and charge = +2)
Charge +2, mass 4 (42He) high specific ionization, limited penetration,
come only from high z (# of protons) atoms.
22688Ra
--> 22286Rn + 42He + energy
23892U -->
23490Th + alpha + 4.19 MeV
Radionuclides which emit alpha are
changed into another nuclide with a mass of 4 units less and 2 fewer protons
Three sheets of paper are
sufficient to stop alpha radiation.
·
when an alpha
particle loses energy it attracts electrons and becomes a neutral helium atom.
·
not used in plant
biology and soil studies.
2. Beta "negatron" (high neutron:proton ratio, originates from the
nucleus like alpha)
·
neutron in the
nucleus changes to a proton, increasing the atomic # by one.
3215P ---> 3216S + B- + e- + v(+1.71 Mev)
3. Beta "positron" (low neutron:proton ratio, comes from the nucleus
which has too many protons)
·
proton in the
nucleus changes to a neutron, decreasing the atomic number by one.
3015P ---> 3014Si + B+ + e+ + v(+3.3 Mev)
4. Neutrino
B. Photons (a quantum of radiant energy)
1. Gamma, does not have a mass
(electromagnetic radiation with the speed of light)
·
is not a mode of
radioisotope decay but rather associated with particulate emission.
·
can penetrate
inches of lead
6027Co ---> 6028Ni + B- +gamma
+ gamma
0.31MeV 1.17 MeV 1.33
MeV
Radio isotope decay schemes result in transmutation of
elements that leave the nucleus in a suspended state of animation. Stability is reached by emitting one or more
gamma photons.
2. X-ray emitting by electron capture (too many
protons and not enough neutrons)
·
emitted when
cathode rays of high velocity fall directly on a metallic target (anticathode)
in a vacuum tube.
·
highly penetrating
electromagnetic radiation (photons) with a short wave-length.
·
identical to
gamma rays if their energies are equal
·
electron from K
ring is pulled into the nucleus
·
chain reaction of
K ring pulling electron into K from L and so on.
·
emission as an x-ray
is external to the nucleus (come from the outer shell of the atom)
3. Cosmic radiation (radiation from outer space)
·
mixture of
particulate radiation (neutrons) and electromagnetic radiation.
________________________________________________________________
Source
of
Radiation
________________________________________________________________
specific
ionization penetration charge nucleus
alpha high low +2 inside 226Ra, 238U, 242Pu*
beta (negatron) medium med +1 inside
beta
(positron)@ medium med -1 inside 90Sr, 32P
gamma low high none inside 60Co
X-ray high outside 59Ni
________________________________________________________________________________
* -
naturally occurring
@ -
characteristic of the majority of radioisotopes used in biological tracer work
Measurement:
A.
ionization takes
place in an enclosed sensitive medium between two oppositely charged electrodes
(ionization chambers, Geiger-Muller)
B.
systems that do not
depend on ion collection but make use of the property that gamma-ray photons
(also alpha and beta) have for exciting fluorescence in certain substances
(scintillation)
C.
ionizing
radiations affect the silver halide in photographic emulsions which show a blackening
of the areas exposed to radiation (autoradiography)
Geiger-Muller Counter: (positron) will not measure gamma.
G-M tube filled with Ar or He.
Ionizing radiation passing through the gas in the tube causes electrons to be removed
from the atoms of gas; form ion-pairs (pairs of electrons and positive
ions). Under the influence of an applied
field, some of the electrons move towards the anode and some of the positive
ions towards the cathode. Charges
collect on the electrodes and initiate pulses; a continuous stream of these
pulses constitute a weak electric current.
Mass Spectrometer:
Positive ions are produced from
molecules or atoms by subjecting them to an electric discharge or some other
source of high energy. The positive ions
are accelerated by means of an electric field and then passed through a slit
into a magnetic field. The slit serves
to select a beam of ions. The charged
particles follow a curved path in the magnetic field which is determined by the
charge to mass ratio of the ion. When
two ions with the same charge travel through the tube, the one with the greater
mass will tend to follow the wider circle.


Block diagram of a double collector mass spectrometer (Vose, 1980)
Scintillation: (alpha, positron,
negatron, gamma)
When certain materials (zinc
sulfide) are exposed to gamma photons or particulate radiation they emit
scintillation's or flashes of light. The
scintillation's are produced by a complex process involving the production of
an excited (higher energy) state of the atoms of the material. When the orbital electrons of these atoms
become deexcited, the excess energy is then given off in an infinitely small
time as a flash of light (scintillation).
Autoradiography:
Radiation Levels:
Limits: 1/10 Rad/week
X-ray
(dentist) 1-5 rads
0-25
rads no injury
25-50
rads possible blood
change, shortened life span
50-100
rads blood changes
100-200 definite injury
(possibly disabled)
200-400 definite disability,
possible death
400-600 50% chance of dying
>600 assured fatal
Radiation
Treatment:
1.
Nucleic acid
injections: enhance blood manufacturing capabilities of the body (blood cells
affected most)
2.
Bee sting venom
(has R-SH radical)
3.
Mercaptan
There
are four stable or heavy isotopes of potential interest to researchers in soil
and plant studies (18O, 2H, 13C and 15N)
Nitrogen
15N
(N2 gas
bombarded by electrons) N2 gas
(cryogenic distillation of nitric
oxide) (microdiffusion techniques)
1.
non radioactive
2.
no time limits on
experiment (versus half-life problems associated with radioactive materials)
3.
less sensitive
than for measuring radioactive elements where we can accurately determine 1
atom disintegrating
4.
mass spec needs 1012 atoms before it can be measured
5.
mass spectrometry
is more complicated.
6.
high enrichment
needed in agricultural work
7.
high cost
associated with purchasing this isotope $250/g
8.
need 3/10
enrichment for 1 year experiments.
9.
discrimination of
plants for 14N versus 15N
10. more sensitive than total N procedures
Nitrogen: radioactive isotopes of N have extremely
short half-lives to be of significant use in agriculture (13N t½ =603 seconds)
% present in
N2 atmosphere
_____________________
14N 14N 99.634
15N 14N 0.366
Ratio needs to be established
before starting the experiment: (e.g., background levels)
100g 15NH415NO3 5%
enriched $200
100g 15NH415NO3 10%
enriched $400
Instead of the specific activity
of a sample used in the case of radioisotopes, the term % abundance is used for
stable isotopes. The % 15N abundance is the ratio of 15N
to 15N + 14N atoms
Because the natural environment
has an 15N abundance of 0.3663%, the amount of 15N
in a sample is expressed as %15N atom excess over the natural abundance of 0.3663. (subtracting
0.3663 from the determination of 15N abundance to obtain
15N
atom excess).
mass spec: detection to 0.002 atom
excess:
Essentially measuring the
intensity of ion currents (R)
R = 14N 14N/15N
14N
% 15N abundance =
100/2R + 1
By measuring the height of the 14N 14N and 15N 14N
peaks (corrected for a background reading), the R values are determined and the
% 15N abundance calculated.
Sample
Preparation:
N in plant and soil samples must
first be converted into N2 gas.
1. Kjeldahl digestion - distillation
into acid - total N determined by titration - aliquot taken for transformation
into N2 gas (Rittenberg Method)
2NH4Cl + 3NaBrO* +
2NaOH ----> N2 + 5H2O
+ 3NaBr + 2NaCl
*alkaline sodium hypobromite
(Vose, p 156)
2. Dumas method (sample heated
with CuO at high temperatures (> 600°C) in a stream of purified CO2 and the gases liberated are led over hot Cu to reduce
nitrogen oxides to N2 and then over CuO to convert CO to CO2. The N2-CO2 mixture thus obtained is collected in a
nitrometer containing concentrated alkali which absorbs the CO2 and the volume of N2 gas is measured.
ERRORS/DILUTION:
1. N in grain, N in tissue
2. N in organic fractions
(immobilized)
3. Inorganic soil N
4. Plant N loss
5. N leaching
For analysis by mass spectrometer,
the analytical error including sub-sampling is approximately 0.01% 15N atom excess for a single sample. Improved instrumentation has taken this to
0.002% 15N atom excess.
Therefore samples should contain
at least 0.20 % 15N atom excess. (5% error)
1% atom excess 15N is adequate for fertilizer experiments where the
crop takes up a substantial portion of the applied fertilizer.
30-50% atom excess is required for
soils experiments where turnover processes are high and where various fates of
N exist (plant N loss, leaching, plant uptake, grain uptake, etc.). For this reason, 15N studies are usually small due to the price.

If 80 kg N/ha are to be applied in an experiment
where the total N uptake is likely to be 100 kg N/ha and the expected
utilization of N fertilizer were 30 %, then 0.33 kg/ha of 15N is required (Vose, p. 165, using Figure X from Fried
et al.).
Therefore, the enrichment required
for a rate of application could be as low as 0.41% 15N atom excess (0.33/80 * 100)
Enriched 15N:
materials with a greater than
natural concentration of 15N
% plant N derived from fertilizer
= %15N
excess in sample
%
15N excess in fertilizer
Depleted 15N:
materials with a lower than
natural concentration of 15N (0.003 - 0.01 atom % 15N)
or (< 0.01 atom % 15N)
·
use of isotopic 14N
·
studies involving
residual soil nitrogen are not practical with depleted materials due to the
high dilution factor.
% plant N derived from the
fertilizer =
(Nu
- Nt)/(Nu - (Nf/n))
Nu =atom % 15N in unfertilized plants
Nt = atom % 15N in fertilized plants
Nf = atom % 15N in the fertilizer (for example 0.006%)
n = the plant discrimination
factor between 14N and 15N.
If it is assumed that there is no
discrimination between 14N and 15N, then n = 1.
Fertilizer N Recovery (Varvel and
Peterson, 1991)
1. Difference method
PFR = (NF)-(NC)
R
NF = total N uptake in corn from N
fertilized plots
NC = total N uptake in corn from
unfertilized plots
R = rate of fertilizer N applied
PFR = percent fertilizer recovery
2. Isotopic method (Depleted
material)
PFR = (NF) x (C-B)/D
R
NF = total N uptake in corn from N
fertilized plots
B = atom % 15N of plant tissue from N fertilized plots
C = atom % 15N of plant tissue from unfertilized plots (0.366%)
D = depleted atom % 15N in applied N fertilizer
R = rate of applied 15N-labeled fertilizer
3. Isotopic method (Enriched
material, Sanchez et al., 1987)
F = As-Ar/Af-Ar
F= fraction of total N uptake
derived from 15N enriched fertilizer
As = atom % 15N measured in the harvested plant sample
Af = atom % 15N in the enriched fertilizer
Ar = atom % 15N of the reference harvested plant material from non 15N
enriched fertilizer treatments
Ef = F x total N uptake
Ef = uptake of 15N enriched fertilizer
Shearer and Legg (1975) found that
d15N of wheat plants decreased as the N application rate
increased.
d15N = atom % 15N
(sample) - atom % 15N (standard)
x 1000
atom % 15
N (standard)
15N composition of the total N of
grain and leaf samples of corn (Zea
mays L.) decreased systematically as N fertilizer rates increased
(Kohl et al., 1973). This result was
considered to be consistent with increasing contributions of fertilizer N to
plants as the rate of applied N increased.
Hauck and Bremner, 1976
percent nitrogen recovered (plant
or soil) =
= 100P (c-b)
f(a-b)
P = total N in the plant part or
soil in kg ha-1
f = rate of 15N fertilizer applied
a = atom percent 15N in the labeled fertilizer
b = atom percent 15N in the plant part or soil receiving no 15N
c = atom percent 15N in the plant part or soil that did receive 15N
unlabeled N uptake = (total N
uptake in grain and straw) -
[N
rate(% recovery of 15N in grain and straw)]
half-life: time required for half of the radioactive atoms to
undergo decay (loss of half of its radioactivity)
32P (t½ = 14.3 days)
14C (t½ = 5568 yrs)
l: Decay constant (fraction of the number of atoms of a
radioisotope which decay per unit time)

A: Activity (decay intensity which
is proportional to the number of radioactive atoms present)
N: number of radioactive atoms
present at time t and
l is the decay constant
l = 0.693/t½
N = No e -lt
A = lN
N for 1 g of pure 32P = 6.025 x 1023/32 atoms/g
= 1.88 x 1022 atoms/g
Isotope Effects:
All tracer studies assume that the
tracer behaves chemically and physically as does the element to be studied (tracee).
Discrimination
of the plant /soil microflora
Isotopic
Exchange (42K , cytoplasm, exclusion K2SO4,
KCl)
Phosphorus 32P
1.
mobile in the
plant
2.
found to
concentrate in the grain
3.
mobility of P in the
plant allows for increased concentration in younger tissue and fruiting bodies.
4.
strong beta
emitter resulting in acceptable characteristics for autoradiograph
techniques.
Agronomic uses:
1.
P use efficiency
2.
Method of placement
3.
P fixation
In general, 32P is no longer useful after approximately 7 half lives
or 100.1 days.
EXAMPLES:
1. What will the activity of 5 mC 32P in 5 ml be in 36 days?
N = No e -lt
A = Ao e -lt
l = 0.693/t½ = 0.693/14.3 = 0.04846
t = 36 days
-lt = 1.744
e -lt = 0.1748
A = 5 mC/5ml * 0.1748
= 0.1748 mC/ml
2.
You intend to set up a field experiment for evaluating the P delivery
capacity of a given soil.
a.
P rate= 18.12
kg/ha (18120 g/ha)
b.
Crop will utilize
10 % of that applied.
c.
Need a count of 1000
cpm at the end of the experiment.
d.
Instrument has a
20% counting efficiency for 32P.
e.
A 10 gram sample
will be used from a total plot weight of 3628 kg/ha.
10/3628000
= 0.000002756
What should the specific activity of
the fertilizer be in mC/g P if 110 days will lapse between planting and sample
assay?
1000 cpm = Ao e -lt
1000 cpm = Ao * e -(0.693/14.3)(110)
1000 cpm = Ao e -5.33
Ao = 1000/0.0048403 = 2.06596 x 105 cpm
2.0659 x 105 cpm ÷ 60 sec/min = 3.443 x 103 dps
3.443 x 103 dps ÷ 0.10 (crop utilization efficiency) = 3.443 x 104 dps
3.443 x 104 dps ÷ 0.20 (counting efficiency) = 1.7216 x 105 dps
1.7216 x 105 dps ÷ 0.000002756 (dilution) = 6.2468 x 1010 dps
6.2468 x 1010 dps ÷ 3.7 x 107 dps/mC (constant) = 1.688 x 103 mC
1.688 x 103 mC ÷ 18120 g = 9.317 x 10-2 mC/g P
3.
How much 32P would you put into a system to assure 500 cpm after
2 months using an instrument with a 10% counting efficiency and 10% P
utilization efficiency?
A = Ao e -lt
500 cpm = Ao * e -(0.693/14.3)(60)
Ao = 500/0.0546 = 9.157 * 103 cpm
9.157 * 103 cpm ÷ 0.10 (crop utilization efficiency) = 9.157 * 104 cpm
9.157 * 104 cpm ÷ 0.10 (counting efficiency) = 9.157 * 105 cpm
9.157 * 105 cpm ÷ 2.22 x 109 cpm/mC (constant) = 4.13 x 10-4 mC
1 mC 32P weighs 3.5 x 10-9 g
4.13 x 10-4 mC x 3.5 x 10-9 g/mC = 1.44 x 10-12 g 32P
Absorption: interception of radiant energy or sound
waves
Adsorption: adhesion in an extremely thin layer of
molecules to the surfaces of solid bodies or liquids with which they are in
contact.
Soils containing large amounts of mineral clay and
organic matter are said to be highly buffered and require large amounts of
added lime to increase the pH.
Buffering capacity (BC): represents the ability of the
soil to re-supply an ion to the soil solution.


You should never use a buffered solution (fixed pH)
for CEC. If a 1 N NH4OAc
solution were used to displace the cations on the exchange complex of a soil
with a pH of 5.0, CEC would be overestimated as pH dependent charge sites would
be included (specifically organic matter) that would not have been present at
the soils natural pH.
Ions must exist in soils as solid compounds or
adsorbed to cation/anion exchange sites.
Can be described by the ratio of the concentrations of
absorbed (D Q) and solution (D I) ions; BC = D
Q/D I
The BC in soil increases with increasing CEC, organic
matter and other solid constituents in the soil.
For most minerals the strength of cation adsorption or
lyotropic series is:
Al+++>Ca++>Mg++>K+=NH4+>Na+
ions with a higher valence are held more tightly than
monovalent cations (exception, H+)
Al+++>H+>Ca++>Mg++>K+=NH4+>Na+
The degree of replaceability of an ion decreases as
its dehydrated radius increases. Cations
are attracted toward, and anions are repelled from, negatively charged soil
colloids. These interactions follow
Coulomb's law where;
F=qq'/Dr2
F is the
force of attraction or repulsion
q and q1
are the electrical charges (esu, equal to 2.09 x 109 individual electronic charges)
r is the
distance of charge separation (cm)
D is the
dielectric constant (=78 for water at 25°C)
The strength of ion retention or repulsion increases
with increasing ion charge, with increasing colloid charge and with decreasing
distance between the colloid surface and either the source of charge or the
soluble ion.
Interaction between ions increases with concentration
and with the square of the ion charge.
The parameter embracing the concentration and charge effects is the
ionic strength (I) of the solution.
I = ½ sum Mi Zi2
where M is the molarity, Z is the charge of each ion
i.
Ionic strength measures the effective ion
concentration by taking into account the pronounced effect of ion charge on
solution properties. A solution has only
one ionic strength but each of its constituent ions may have a different
activity coefficient.
Exchangeable bases: Ca++ Mg++ K+ and Na+
Exchangeable acidity:
1. H ions obtained from the hydrolysis of
exchangeable, trivalent Al
2. Hydrolysis of partially hydrolyzed and nonexchangeable
Al
3. Weakly acidic groups, mostly on organic matter
4. Exchangeable H
In
the early days of soil science there was no agreement on the pH of the soil at
which exchangeable acidity was to be determined. Bradfield, 1923 noted that the usual
substance used to increase the pH of acid soils is CaCO3 and that the maximum pH obtainable with CaCO3 is pH 8.3.
Therefore base saturation is defined as the quantity of base adsorbed by
a soil in the presence of CaCO3 equilibrated with air having a CO2 content of 0.03% (Thomas, 1982).
Cation Exchange Capacity (CEC):
1.
Sum total of
exchangeable cations on the exchange complex expressed in meq/100g (Ca++,
Mg++, K+, Na+, H+, Al+++)
2.
Quantity of
readily exchangeable cations neutralizing negative charge in the soil
3.
Exchange of one
cation for another in a solution phase
4.
Soils capacity to
adsorb cations from an aqueous solution of the same pH, ionic strength, dielectric
constant and composition as that encountered in the field.
Extract sample with neutral 1 N ammonium acetate. (NH4OAc)
·
exchange
complex becomes saturated with NH4
·
extract same soil
with 1N KCl (different salt solution), K+ replaces NH4
·
quantity of
ammonium ions in the leachate is a measure of CEC
example:
-filtrate has 0.054 g of NH4
(20 g of soil extracted)
1 meq of NH4 = (14+4)/1000
= 0.018g/meq or 18g/eq
0.054/0.018 = 3 meq
3 meq/20g = 15meq/100g
increase
clay, increase CEC
increase
increase
2:1 clays, increase CEC
1:1
clays: 1-10 meq/100g
2:1
clays: 80-150 meq/100g
Extraction
with an unbuffered salt which would give a measure of the CEC at the soils
normal pH.
Use
of neutral N ammonium acetate (7.0) will result in a high CEC on acid soils
because of the adsorption of NH4 to the pH
dependent charge sites.
Why?
1.At
high pH, H+ are weakly held and may be exchanged; pH dependent
charge
2.Deprotonation
(dissociation of H from OH groups at the broken edges of clay particles which
is the prime source of negative charge in 1:1 clay minerals) occurs only at
high pH (7.0 and up)
Kamprath:
unbuffered salt solution, 1.0 N KCl will extract only the cations held at active
exchange sites at the particular pH of the soil. The exchangeable acidity is due to Al and H.
1.
Presence of CaCO3 and/or CaSO4 (dissolution) and the presence of salt in arid
type soils. Dissolution of CaCO3 and/or CaSO4 will cause Ca to exchange for Mg, K and Na
instead of NH4 replacing all
of these. When 1 N KCl is then added to
displace the NH4 (from NH4OAc)
less NH4 is detected in
the filtrate than what should have been present.
2.
Variable charge
soils (high content of more difficult exchangeable aluminum-hydroxy
"cations"). Exchangeable Al
and its hydroxy forms are not readily exchanged with monovalent cation
saturation solutions. This error results
in an underestimation of CEC.
CEC
Methods
1.Polemio & Rhoades (1977) arid soils containing
carbonates, gypsum and zeolites.
a.
Saturation of
exchange sites with Na (pH 8.2) 0.4N NaOAc + 0.1N NaCl
b.
Extraction with
0.5N MgNO3
c.
Na determined
(soluble Na from saturation step deducted from total Na to obtain exchangeable Na)
d.
Method will
determine CEC as a result of permanent charge but not for variable charged
soils (pH)
2. Gillman (1979) acid soils
a.
Saturation of
exchange sites with BaCl2 (solution of a concentration approximately equivalent
in ionic strength to the soil solution)
b.
Extraction with
MgSO4 to replace Ba with Mg (MgSO4 concentration is adjusted to achieve an ionic
strength comparable with that of the soil solution)
c.
Ba determined
The
use of unbuffered solutions throughout ensures that natural soil pH is not
significantly altered.
The
underlying factor which has caused various researchers to develop alternative
methods for determining CEC was how to deal with pH dependent charges (pH of
the saturating solution and replacement solution). This is important considering the pH is a
logarithmic function of H+ where 10 times as much H occurs in solution at pH 5
as pH 6.
Reflects
the extent of leaching and weathering of the soil.
It is
the percentage of total CEC occupied by cations, Ca++, Mg++, Na+ and K+, where each is
determined separately from the NH4OAc extract (Atomic
Absorption - interception of radiant energy)
Amount
present in soil
Ca
0.03g
Mg
0.008g
Na
0.021g
K
0.014g
Meq
of each cation (amount present/g per meq)
Ca =
0.03/0.02 = 1.5
Mg =
0.008/0.012 = 0.66
Na =
0.021/0.023 = 0.91
K =
0.014/0.039 = 0.36
=3.43meq/20g
=17.15 meq/100g
CEC =
20 meq/100g
BS =
17.15/20 = 85.85%
BS =
CEC - (H+ + Al+++) / CEC * remember this is exchangeable H+ and Al+++
pH
and BS are positively correlated
Why
would pH and BS be positively correlated if pH and CEC were not?
Adsorption
of anions to + charged sites in hydrous oxide minerals where the hydrous oxides
are amphoteric (have - and + charge depending on pH and therefore have AEC and
CEC).
Order
of adsorption strength H2PO4- > SO4= >NO3- = Cl-
pH
< 7.0
More
in weathered soils (1:1) containing hydrous oxides of Fe and Al (exposed OH
groups on the edges of clay minerals)
Soils
which have pH dependent charges.
Anion
exchange of 43meq/100g at an acidic equilibrium pH of 4.7.
Can a
soil have a net positive charge?
(unlikely)
Is H2PO4- adsorption on soils anion exchange? yes
only
physically adsorbed initially but soon precipitate as Ca-P in alkaline soils
and Fe or Al-P in acid soils.
Can P
applications induce S deficiencies in acid soils?
Acid
soil: S levels low --> P exchange for S on exchange complex (anion exchange)
and SO4= can be leached.
90%
of all water soluble bases will be leached as sulfate (Pearson et al, 1962)
Kamprath
et al. (1956)
1.
Increased P
concentration in solution reduced the amounts of SO4= adsorbed by the soil.
2.
Amount of sulfate
adsorbed decreased as the pH of the soil suspension increased (4 to 6).
Aylmore
et al. (1967)
1.
Sulfate
adsorption on clays possessing positive edge charges + oxides of Fe and Al
(highly resistant to leaching and less available for plant growth)
2.
Sulfate adsorbed
on kaolinite clay is weakly held and easily released
Fox
et al. (1964)
Ca(H2PO4)2 best extracting
solution for S
AEC negatively correlated with Base Saturation
Ca10(PO4)6(OH)2
Hydroxyapatite
Ca10(PO4)6F2 or Cl2
or OH2
Fluorapatite
27-41% P2O5
P
fertilizers:
1.
water soluble
2.
citrate soluble
(dissolves more P than water)
OSP
ordinary superphosphate (0-20-0)
·
rock phosphate +
sulfuric acid
·
mixture of
monocalcium phosphate and gypsum
·
16-22% P2O5 (90 % water
soluble)
·
8-10% S as CaSO4
TSP
triple or concentrated superphosphate (0-46-0)
·
rock phosphate +
phosphoric acid
·
essentially all
monocalcium phosphate
·
44 to 52% P2O5
(98% water soluble)
·
< 3% S
·
major phosphate
mineral is monocalcium phosphate monohydrate (MCP)
DAP
Diammonium phosphate (18-46-0)
·
Reacting wet
process H3PO4 with NH3
·
46-53% P2O5
MCP monocalcium
phosphate monohydrate Ca(H2PO4)2 2H2O
(highly water soluble)
DCPD dicalcium
phosphate dihydrate CaHPO4* 2H2O - brushite
DCP dicalcium
phosphate CaHPO4, 53% P2O5 - monetite
congruent
dissolution of Ca(H2PO4)2 2H2O
into Ca++ and H2PO4 ions occurs at a pH of 4.68
Examples:
1.
P deficient
2.
S deficient
3.
pH 5.5
4.
anion exchange 20
meq/100g
·
Apply triple
superphosphate with gypsum
·
Supersaturate the
band with respect to Ca and precipitate P as DCP and or DCPD which will be
slowly available with time.
Lindsay
(1979)
·
including NH4+,
K+, Ca++ and Mg++ enables these cations to be
included in the initial reaction products.
·
MCP contains
sufficient Ca to precipitate half of P as DCPD or DCP.
·
In acid soils, Fe
and Al generally precipitate the additional P.
·
Avoid anion
exchange interaction (P displacing S from the complex)
Low
Soil pH (<5.5) P precipitates as Al and Fe phosphates
a. variscite (ALPO4 * 2H2O)
b. strengite (FePO4 * 2H2O)
Moderate
to High pH, P precipitates as Ca phosphates (several)
a. dicalcium phosphate (CaHPO4)
b. dicalcium phosphate dihydrate
(CaHPO4 * 2H2O)
c. hydroxyapatite Ca5(PO4)3OH
d. fluorapatite Ca5(PO4)3F
(rock phosphate)
Precipitation
- Dissolution of phosphate minerals is pH dependent:
Precipitation/Dissolution
can be determined by using P solubility diagrams.
1. Soil solution (H2PO4-) and pH above the line (precipitation)
2. Soil solution (H2PO4- and pH below the line (dissolution)
pH 4.5 Event Precipitate
Formed
1. add fertilizer soluble
P added -
2. 1 - 2 soluble
P decreases DCP
3. 2-3 DCP
dissolves FA
4. 3-4 FA
dissolves Variscite

Example of precipitation/dissolution (1-4)
Can P fertilizers be used as a source of Lime? if enough is applied, yes, but this will not be economical
8. Theoretical Applications in Soil Fertility
1.
Liebig's law of
the minimum
2.
Bray's Nutrient
Mobility Concept
3.
Sufficiency
(SLAN)
4.
Mitscherlich
5.
Bray modified
Mitscherlich
6.
Base Cation
Saturation Ratio
Liebig's law of the minimum (Justus von Liebig 1803-1873)
He
stated that the nutrient present in least relative amount is the limiting
nutrient.
soil contained enough N to produce
50 bu/ac
soil contained enough K to produce
70 bu/ac
soil contained enough P to produce
60 bu/ac
N would be the limiting nutrient.
Crop
used up all of the deficient nutrient in the soil making the yield directly
proportional to the amount of the deficient nutrient present and the crop
content of the nutrient.
Bray Nutrient Mobility Concept

Sufficiency: SLAN (Sufficiency Levels of Available Nutrients)
a.
Range of nutrient
(insufficient to sufficient)
b.
Amount extracted
from the soil is inversely proportional to yield increases from added
nutrients.
c.
Calibrations
exist for the changing levels of available nutrients with fertilizer additions
and yield response.
d.
Concept assumes little
if any effect of the level of availability of one ion on that of another.
e.
Recognizes that
an addition of the most limiting element may cause more efficient utilization
of a less limiting element.
Mathematical
expression of the law of diminishing returns where increases in yield of a crop
per unit of available nutrient decreases as the level of available nutrient
approaches sufficiency.
The
concept is based on Mitscherlich's equation:
dy/dx
= (A-y)c
Yield
increases (dy) per unit of available nutrient (dx) decrease as the current
yield (y) approaches a maximum yield (A) with c being a proportionality
constant.
The
derivative was developed for studying tangent lines and rate of change. The first derivative is the slope of the
tangent line at xo
d/dx
xn = nxn-1
Quadratic:
Y = bo + b1x - b2x2
0 = b1-2b2x
2b2x
= b1
x=b1/-2b2
Plant Response to Soil Fertility as Described by the Percent Sufficiency and the Mobility Concept
Simply stated, plants respond to the
total amount present of mobile nutrients and to the concentration present of
immobile nutrients in soils. Stated this
way, yield is directly related (proportional) to the total amount of nutrient
present in the soil. However, yield
response to immobile nutrients is not related to the total amount of the
“available form” present in the soil, but instead is a function of the concentration
of available form at, or very near, the
root surface. Ideally, the response of
crops to mobile nutrients should be linear because mobile nutrients (like
water) are not decreased in availability by reaction with the soil. The linear response to mobile nutrients
continues with each added increment of the nutrient until the yield potential
for that growing environment has been reached, after which it is zero (see
figure below)

The
ideal situation is not found in soils, only in hydroponics and when the
physical phase of the growth media is not reactive, such as with glass
beads. However, because the reaction of
some nutrients with soil is sometimes minimal (e.g. nitrate-N in cultivated
soils with minor potential for immobilization and mineralization of N), they
are considered relatively mobile and response tends to follow the ideal. Hence, “rules of thumb” have been developed
to guide the use of mobile nutrients like nitrogen, such as “it takes 2 lbs N/bushel
of wheat”. The 2 lbs is calculated from
the protein or N content (on average) of a bushel of wheat, with the added
assumption that measured soil nitrate-N and added fertilizer N will be only 70%
utilized. Bray’s mobility concept
implies that if available N, for example, is limited to some level below
maximum yield potential then a yield plateau will occur at that point. For example, if there is enough total amount
of available mobile nutrient to produce the yield potential (20 bu.) and then
midway through the season better than average weather conditions result in
increasing the yield potential (to 30 bu.), the mobility concept implies the
yield will be limited to 20 bu. because the total supply of nutrient will be
used up to produce 20 bu. and additional yield can only be obtained if more of
the nutrient is added (this is the reason for top dressing wheat midway through
the season).

For
immobile nutrients, like phosphorus, plants can only extract the nutrient from
soil close to the root surface, very little of the nutrient is moved to the
root by water in the transpiration stream because soil solution concentrations
are minute (< 0.05 ppm for phosphate compared to as high as 100 ppm for
nitrate-N). As a plant grows and roots
extend out into the soil, roots come in contact with “new” soil from which they
can extract phosphate. The amount
extracted is limited by the concentration at (or very near) the root-soil
interface. If the concentration of
phosphate available to the plant at the root -soil interface is inadequate to
meet the needs of the plant, then the plant will be deficient in P throughout
its development. The deficiency will
always be present, and plant growth and crop yield will be limited by the
degree to which the immobile nutrient is deficient. Another, perhaps more common way of
expressing this nutrient limitation is to state that yield will be obtained
according to the sufficiency of the nutrient supply. When this is expressed as a percentage of the
yield possibility then the term percent sufficiency may be applied. Whenever the percent sufficiency is less than
100, plant performance is less than the yield possibility provided by the
growing environment. Consequently, it
does not matter whether the yield possibility is 20 bu. or 30 bu., if the
percent sufficiency is 80, then actual yield obtained (theoretically) will only
be 80% of that yield possibility.
The soil test for mobile nutrients
is an indicator of the total amount available.
If this amount is enough to produce 20 bu/ac, more N would have to be
added to the total pool to produce 40 bu/ac.
With P, an index is developed that is independent of the
environment. If the crop year was good,
roots would expand into more volume of soil that had the same level of nutrient
supply. Sufficiency is independent of
the environment since increased root growth will expand into areas where
contact exchange uptake is the same (total amount present in the soil is not
greatly affected).
Concept yield goal sufficiency
Environment dependent independent
Sorption
Zone root system root surface
Influence
of
crop uptake
on total available large small
Soil test is an
indicator of the
total available yes no
Soil
solution
concentrations 0-100 ug/g <0.05
ug/g
Function
of conc. in the root
syst. conc. at the root surf.
Topdress
appl. Yes No
____________________________________________
Example:
Wheat
(4081 kg/ha = 60 bu/ac)
2.5%N in the grain
=102.03 kg N
(4081 kg/ha = 60 bu/ac)
0.36%P in the grain
=14.69 kg P
Soil
0.1%
N*10000=1000 ug/g * 1.47 * 1.524 = 2240
kg N/ha 0-15 cm
NO3-N:
10 ug/g * 1.47 * 1.524 = 22.40 kg NO3-N/ha
0-15 cm
NO3-N
soil test is the actual N available at time X
NO3-N
soil test is valid for one point in time (1 crop or year)
Some states predict
N mineralization
0.1%
P*10000=1000 ug/g * 1.47 * 1.524 = 2240
kg P/ha 0-15 cm
P soil test is an
index (sufficiency) of availability
P soil test is
valid for up to 5 years or more**
10 ug/g P, Mehlich
III is not equal to 22.40 kg P/ha
We cannot predict P
mineralization
(102.03/2240)*100
= 4.5%
(14.69/2240)*100
= 0.65%
Steps for Using the Sufficiency Concept:
1.
Selection or
determination of the sufficiency level
a.
estimated from
results of studies with a crop on similar soils.
2.
Computation of
fertilizer required for sufficiency
a.
amount of soluble
P required to raise the available P from the initial level to the sufficiency
level.
3.
Method of
supplying the fertilizers (and/or lime)
a.
soil build-up
plus crop needs (BUILD-UP) long-term.
b.
crop needs
(MAINTENANCE) short-term.
Mitscherlich-Baule
percent sufficiency concept:
When
more than one nutrient was deficient, the final percent sufficiency is the
product of the individual sufficiencies.
_____________________________________________________
Maximum
yield when N,P and K are
present in sufficient quantities 5000 kg/ha
Yield
when N and P are present in
sufficient quantities 4000 kg/ha
4000/5000
= 80% of MAXIMUM
Yield
when N and K are present in sufficient
quantities 3000
kg/ha
3000/5000
= 60% of MAXIMUM
_____________________________________________________
What
will be the predicted yield when only N is present in
sufficient
quantities 2400
kg/ha
5000(0.6
* 0.8)
_____________________________________________________
"present"
function of both soil levels and amount applied.
If
this percent sufficiency concept is correct, then Liebig's concept of the
limiting nutrient is wrong.
Sufficiency
Calculations
________________________________________________________________
Present Field X Field Y * Field Z *
in adequate Yield kg/ha
amounts
________________________________________________________________
NP 6400 9600 8000
12000*.8
NPK 8000 12000 10000
8000/.8
NK 7200 10800 9000
12000*.9 10000*.9
PK 7000 7000 7000
________________________________________________________________
N 5760 8640 7200
8000*.8*.9 12000*.8*.9 10000*.8*.9
________________________________________________________________
% sufficiency K NP/NPK = 6400/8000 = 0.8
% sufficiency P NK/NPK = 7200/8000 = 0.9
* - assume that the % sufficiency levels for P and K are the same in field Y and field Z
_____________________________________________________
Leibig's law of the minimum: correct for mobile nutrients
Mitscherlich: correct for immobile nutrients.
Mitscherlich was incorrect in his use of c values for N 0.122.
When the value of c is small a large quantity is needed and visa versa.
Mitscherlich (applicability of this growth function to soil test correlation studies)
The
original work by Mitscherlich showed that the response of plants to nutrients in the soil can be expressed by a curvilinear
function and a logarithmic equation, and further concluded that the regression
coefficient c in the equation was constant for each nutrient regardless of any change in
environment, plant type, soil and other factors (Balba and Bray, 1956).
log
(A-y) = log A - cx
A =
yield possibility when all nutrients are present in adequate amounts but not in
excess
y =
yield obtained at a given level of x (dy = dx) and when y is always less than
A(99%)
c =
proportionality constant
NOTE:
some texts use c and others c1, however, it does not matter which
one is used, so long as they are defined.
Similarly, b and x are used interchangeably
dy dy
---- = c(A-y) and ----- =
dxc
dx (A-y)
log(A-y) = log A - cx
* A
and y can be expressed as actual yield or % of the maximum yield
STEP 1.
Experimental locations with different soil test P (b)
levels
NPK NK Sufficiency x calc. c
Loc 1 30 20 0.66 12 1.53=2-12c 0.039
Loc 2 40 15 0.375 4 1.79=2-4c 0.051
Loc 3 30 16 0.53 9 1.67=2-9c 0.036
avg. 0.042
A=100
y =
66
x =
12
log(100-66)
= log 100-12c
1.53
= 2 - 12c
12c
=0.47
c
= 0.039
STEP 2.
Apply
value of c where applicable. If the soil
pH or soil test K changes over an area, then c has to be altered accordingly.
Now
that an average c factor has been determined, we can relate the soil test level
of b with yield sufficiency for this element.
(CAN determine % SUFFICIENCY)

STEP 3. (Bray Modified Mitscherlich)
Expand
Mitscherlich to calculate the amount of fertilizer needed to raise the percent
yield from any given starting level to any other desired upper level for which
fertilization is desired
Log(A-y)
= log A - cb - c1x
c1
= efficiency factor for the method of applying the fertilizer (determined from
fertilizer studies). This factor will
change accordingly for immobile nutrients (band versus broadcast)
x =
quantity of fertilizer that needs to be applied.
STEP 4.
Fertilizer
studies c1 (broadcast
P) = 0.0070
c1
(banded P) = 0.0025
c
and c1 vary with
1. crop
2. planting density/pattern
3. nutrient applied (source)
4. method of application
5. management
6. soil
Yield
Possibility
1. soil
2. climate, moisture
3. yield potential (hybrid)
4. planting density and pattern
Soil
Nutrient Requirement (level determined)
1. when sampled
2. stage of growth
3. crop
4. form of nutrient applied
5. analytical method
Fertilizer
Requirement (x)
1. b
2. fertilizer used
3. crop
4. placement
Log
(A-y) = Log A - cb - c1x
A =
maximum yield
y =
yield obtained at some level of b
b =
soil test index
c =
efficiency factor (constant) for b
x =
amount of fertilizer added to the soil
c1
= efficiency factor for x (method of placement)
Example:
Soil test value for P = 20
N, K
and all other nutrients adequate
kg
P/ha Yield, kg/ha % Sufficiency
0 2000 40
25 3000 60
50 4500 90
75 5000 100
log (100-40) = log 100 - c(20)
1.778 = 2.00 - c(20)
-0.2218 = -c(20)
c = 0.01109
solve
for c
log
(5000 - 3000) = log 5000 - 0.01109(20) - c1(25)
3.301
= 3.477 - c1(25)
c1
= 0.00704
log
(5000 - 4500) = log 5000 - 0.01109(20) - c1(50)
2.6989
= 3.477 - c1(50)
c1
= 0.0155
average
of c1 = (0.00704 + 0.0155)/2
=
0.011303
STEP 5:
Apply
concept (solve for x, determine the amount of fertilizer to be applied)
Log
(A-y) = log A - cb - c1x
* The
dangers of using % yield: It is difficult to determine amounts of fertilizer to
add (e.g., 2.0 Mg/ha yield and 4.0 Mg/ha yield).
Assumes
that reliable soil test data is available for good soil test correlation.
Assuming that plants take up
nutrients from two different sources in direct proportion to the amount
available, the A-value was developed as the expression
A = B(1-y)/y
where; A = amount available nutrient in the soil
B = amount of fertilizer nutrient
(standard) applied
y = proportion of nutrient in the
plant derived from the standard
"In a true sense, the plant
is the only agent that can determine the amount available."
For a specific soil, crop and
growing conditions, the A-value is constant, and has been found to be
independent of rate of fertilizer application, size of test pot and growth rate.
The A value was primarily
developed to determine the availability of P in soil (P supplying power of a
given soil).
With the band placement, the A
values increased with increasing P rates.
This suggests that the availability to plants when P was banded does not
remain constant with increasing rates.
Fried and Dean (1951) noted that
because it can be assumed that the method of placement does not change the soil
phosphorus, the lower A values obtained with the band placement can be
attributed to a higher availability of the standard (nutrient applied).
For
optimum growth of crops, both a best ratio of basic cations and a best total
base saturation exist in a soil.
Bear
et al. (1945)
Percent
saturation of cations selected as being "ideal". Work originally conducted on alfalfa. Historically, it is interesting to note that
this work was being done at the same time Bray developed the mobility concept.
Ca 65%
Mg 10%
(minimum required for alfalfa)
K 5%
H 20%
Ca:Mg
> 6.5:1
Ca:K
> 13:1
Mg:K
> 2:1
Bear
et al. (1945) suggested that
1.
10% Mg saturation
was minimal for alfalfa
2.
Soluble Mg sources
were essential for correcting Mg deficiencies in sandy soils
3.
Liming above 80%
base saturation (20% H) brought about deficiencies of Mn and other
micronutrients.
Graham
(1959) established ranges or % saturation of the CEC for the 'ideal' soil
Ca:
65-85
Mg:
6-12
K:
2-5
H: ?
·
When this
proportion exists, you can obtain maximum yield.
·
Works only in
sandy soils.
Principles Involved:
1.
Bonding of
cations to exchange sites differs greatly from one type of cation to another
and it differs greatly for the same type of cation at different saturations.
2.
Exchangeable
cations are not proportional to soluble amounts (plant available)
3.
Excess of one
type of cation may depress the activity and plant uptake of another
4.
Adsorbed ion (x)
can have marked effects on the ion in question
5.
Capacity (total
exchangeable) and intensity (activity) of an adsorbed cation influence the
total availability of a cation to the plant
6.
Saturation of pH-dependent
charges increases the activity and plant availability of divalent basic cations
Steps in USING BCSR:
1.
Soil analyzed for
exchangeable bases
2.
Lime required to
raise the soil pH to X
3.
CEC is determined
by totaling basic cations + acidity (exchangeable H and Al), each expressed as
meq/100g or cmol/kg
4.
Each basic cation
expressed as a % of the total CEC
5.
Cations must be
added to the extent that the existing saturations of basic cations = ranges chosen
(e.g., some must decrease and others must increase)
·
Works well on low
to moderate CEC soils and coarse textured soils, highly weathered soils of low
pH that require major adjustments in fertility.
·
Useful where it is
important to maintain a fairly high level of Mg in the soil to alleviate grass
tetany in ruminants.
Grass tetany (low concentrations
of Mg and Ca in cool-season grasses in late fall and early spring).
Grass tetany will occur when
forage contains K/(Ca+Mg) > 2.2
(physiological nutrient imbalance which leads to muscle spasms and deficient parathyroid secretion)
9. Soil Testing / Critical Level Determination
1. Assess the relative adequacy of available nutrients (or lime requirements)
2.
To provide guidance on amounts of fertilizers
(or lime) required to obtain optimum growth conditions for plants (
3. Diagnosis of nutrient limitations before a crop is planted so that corrective measures can be taken.
*Must be fast, reliable and reproducible
PROBLEMS:
Philosophical differences exist on interpreting the tests which result in radically different fertilizer recommendations
1. Base Cation Saturation Ratio
2. Nutrient Maintenance
Disregarding the soil test level, a quantity of nutrient should be added to replace the amount expected to be removed by the crop. All required nutrients- not feasible.
3. Nutrient Sufficiency
No yield response to nutrients above a certain soil test level.
a. response assured very low
b. response likely low
c. response possible medium
d. response unlikely high
Depth of Sampling
1. 0-6, 0-8, 0-12, inclusion of subsoil (micronutrients)
Critical Levels
1. Cate Nelson
2. Mitscherlich
3. Quadratic
4. Square Root
5. Linear-plateau
Economic and Agronomic Impacts of Varied Philosophies of Soil Testing (Olson et al., 1982)
Field experiments (1973-1980)
4 locations
Irrigated Corn (Zea mays L.)
5 soil testing laboratories
No differences in yield
No agronomic basis for 'balance' or 'maintenance' concepts
K, S, Zn, Mn, Cu, B, Mg, Fe
% yield versus soil test level
Two Groups:
1. probability of response to added fertilizer is small
2. probability of response to added fertilizer is large
A. Percent yield values obtained for a wide range in locations (fertilizer rate studies)
· Percent yield = yield at 0 level of a nutrient / yield where all factors are adequate
B. Soil test values obtained (Check Plot)
· Will generate a single % yield and one soil test value for each location
C. Scatter diagram, % yield (Y axis) versus soil test level (x axis)
· Range in Y = 0 to 100%
D. Overlay
· overlay moved to the point where data in the +/+ quadrants are at a maximum
· point where vertical line crosses the x = critical soil test level

depends on the extraction method used and crop being grown.
Maximizes the computed chi-square value representing the test of the null hypothesis that the # of observations in each of the four cells (quadrants is equal).
2. Mitscherlich
3. Quadratic
4. Square Root
5. Linear Plateau: obtaining the smallest pooled residuals over two linear regressions.
Equation MR MER (dy/dx = PR)
________________________________________________________________________________
2. Mitscherlich Log(A-Y) = Log A - C1(x+b) x=log((2.3*A*c)/PR)/c-b
3. Quadratic y = b0 + b1(x) - b2(x2) x=0.5
b1/b2 x=(PR-b1)/(2*b2)
4. Square Root y = bo + b1(x) + b2(sqrt(x)) x=0.25(b2/b1)2 x=(b2/ 2*(PR-b1))2
5. Linear Plateau y = bo + b1(x) when x < joint
y = bo + b1(joint) when x >
joint
________________________________________________________________________________
PR = (price per unit fertilizer) / (price per unit yield)
Optimum rate of fertilizer capable of generating the maximum economic yield is dependent upon the price of fertilizer, the value of the crop and magnitude of fixed production costs. The value of a crop defined as a function of yield and rate of fertilizer can be expressed as:
V = Y * Py = F(x) * Py
where yield (Y) for each fertilizer rate is multiplied by the crop price (Py) per unit of yield. A line describing fertilizer costs per unit area cultivated can be expressed as a function of fixed costs (F) and fertilizer price (Px) times the amount of fertilizer (X)
T = F + Px * X
where total cost (T) is a linear function of fertilizer amount, the slope of the line is given by the price of fertilizer and the intercept by the amount of fixed costs involved (F).
A plot of the value and cost functions illustrates the areas where use of fertilizer is profitable. Net profit can only be generated by use of a fertilizer amount equal or greater than 0-x1. Fertilizer should not be used if the value curve is lower throughout than the total cost curve for fertilizer plus fixed costs (F). With fixed costs involved, the amount of fertilizer that can be used profitably is greater than zero or an amount equal to or greater than 0-x1. For fertilizer input greater than 0-x1, crop value exceeds costs and net profit is generated. Profit from fertilizer application can be increased until input reaches the value of 0-x2. This is the level which maximizes profit. At 0-x2 the difference between value and cost is at a maximum.
For each production function the amount of fertilizer which maximizes profit can be found by obtaining the first derivative and setting it equal to the price ratio (PR).
PR = Price per unit of fertilizer / Price per unit of yield
(from Barreto and Westerman, 1985)

Soil Testing for Different Nutrients
Total Nitrogen in Soils:
Surface soils: 0.05 to 0.10%
precision 0.01% = +/- 200 lb/ac
Why would we run total N on soils if the precision is so low?
· long term experiments (differences greater than 200 lb N/ac)
· C:N relationships at the same level of precision
A. Kjeldahl 1883 (organic + inorganic N)
1. digestion to convert organic N to NH4
2. determination of NH4 in the digest
(N pool consists of NO3-, NH4+, NO2-, organic N)
devardas: reducing agent, that is a finely powdered mixture of metals that act as a source of donor electrons to reduce NO3- and NO2- to ammonium
devardas
N pool + K2SO4, CuSO4, Se, H2SO4 -----> (NH4)2SO4
Digest
(NH4)2SO4 + NaOH ----> NH3 + NaSO4 (catch in boric acid)
titrate
K2SO4 is used to raise the temperature of the digest (increases speed and completeness of the conversion of organic N to NH4)
Se, Cu are used as catalysts to promote the oxidation of the organic matter
NO3 and NO2 are not included in the total N analysis from dry combustion, but it does not matter since there will be less than 20 lb N /ac as NO3 and the total N procedure detects to only +/- 200 lbs N/ac
e.g.
0.01 +/- 200 lbs/ac 20 lbs N/ac as NO3 is lost between 0.01 and 0.02 %total N
0.02 +/- 400 lbs/ac because its small value exceeded the detection limits.
On a KCl extract: (have both NH4 and NO3 in the extract)
1. distill over once (to collect NH4)
2. add devardas alloy (distill over again to collect NO3 and NO2)
devardas alloy: acts as a source of donor electrons to reduce NO2 and NO3 to NH4
problems: N-N and N-O compounds
Sample heated with CuO at high temperature (above 600 °C) in a stream of purified CO2 and the gasses lost are passed over hot Cu to reduce nitrogen to N2 and then over CuO to convert CO to CO2. The N2-CO2 mixture is collected in a nitrometer containing concentrated alkali which absorbs the CO2 and the volume of N2 gas is measured.
2NH4Cl + 4CuO -----> N2 + 4H2O + 2CuCl + 2 Cu (CO2)
problems: heterocyclic compounds (pyridine) are difficult to burn
NA-1500
Sample weighed in a tin (Sn) container
Combustion reactor enriched with pure oxygen (sample oxidation) 1020 °C in combustion tube
Reaches 1700 °C during flash combustion (complete oxidation)
Flash combustion converts all organic and inorganic substances into elemental gases (stable compounds combusted
Combustion products carried by He pass through an oxidation catalyst of Chromium oxide
Combustion
Reactor Reduction Reactor

CO + 1/2O2 = CO2 (Cr2O3 is accepting electrons)
Cr2O3 ensures complete combustion (oxidation) of all organic materials
![]() NOx N2 (Cu is
donating electrons)
NOx N2 (Cu is
donating electrons)
Combustion products (CO, N, NO) and water pass through a reduction reactor (metallic Cu).
Excess O2 is removed in the reduction reactor (Cu at 650 C).
N oxides from the combustion tube are reduced to elemental N2 .
Taking CO, N, NOx and converting them to CO2, N2.
Gases are separated in a chromatographic column and detected using a thermal conductivity detector (TCD) which gives an output signal proportional to the concentration of the CO2 and N2 present.
Rittenberg Method (N2 gas from sample)
2NH4Cl + 3NaBrO + 2NaOH ----> N2 + 5H2O + 3NaBr + 2NaCl
sodium hypobromite
NO3-N
Inorganic N may represent only a small fraction < 2% of the total N in soils (Bremner, 1965)
Nitrate testing does not work in
high
high mineralization potential
consideration of NH4
R-NH2 groups from N cycle
· rapid changes (biological transformations) affect inorganic N analysis
NO3-N and NO2-N
1. Phenoldisulfonic acid or chromotrophic acid
· interference of organic matter, Cl and Fe have affected these colorimetric procedures
2. Selective ion electrodes
· interference of Cl
· (NH4)2SO4, AgSO4 extracting solution: Ag used to precipitate Cl
3. Cadmium reduction
· 2 M KCl extract (colorimetric procedure) - samples are stable for several months if stored at low temperatures
· not subject to interference, extremely sensitive making dilution possible.
· NO3 reduced to NO2 by passing through a column of copperized Cd
· NO2 reacts well with the diazotizing reagent (sulfanilamide) and NO3 does not, thus explaining the need for reducing NO3 to NO2 for analysis using the Griess-Ilosvay method
4. Steam distillation with Devardas alloy (reductant) reduce NO2 and NO3 to NH4
NH4-N
Bremner (1959) stated soils contain a large amount of fixed (non-exchangeable) NH4. Defined as the NH4 that cannot be replaced by a neutral K salt solution present as NH4 ions in interlayer positions of 2:1 type clay minerals.
Air-drying can lead to small but significant changes in NH4-N
1. Steam distillation with MgO (alkaline reagent) color: indophenol blue
2. 2 M KCl (indophenol blue) phenol and NH3 react to form an intense blue color
3. Ammonia gas sensing electrodes
Problems in N analysis:
· -accuracy is measured by the least precise measurement.
· -weight of the soil is the largest error (propagates through to +/- 0.01%N)
0.01% N = +/- 100 ppm (0.01* 10000)
total N in soils 0.10 = 1000 ppm +/- 100 ppm
inorganic N in soils 0.002 = 20 ppm +/- 1 ppm
Total N Inorganic N Organic N?
1000 ppm 20 ppm 980 ppm
1. Inorganic N is not determined on a percent basis because it is done on an aliquot basis.
2. Cannot subtract 20 from 1000 to get organic N (determined on a different basis).
3. Unrealistic because of the incompatibility of error terms.
4. Organic-N is difficult to determine (by subtraction, we have an extremely poor estimate).
Organic N
Procedures exist, but are unreliable and are not reproducible.
Mineralizable N
1.Leach with CaCl2 - dissolves all the soluble N (NO3 and NO2)
2.Incubate the soil - over time - to determine the amount of NO3 that has been mineralized (set period of time under set conditions)
3.Leach with CaCl2 again (sample now has NO3)
4.Determine concentration
Phosphorus Soil Index Procedures
Bray and Kurtz P-1
0.025
Designed to remove easily acid soluble forms of P, largely calcium phosphates and a portion of the aluminum and iron phosphates. The NH4F dissolves aluminum and iron phosphates by its complex ion formation with these metal ions in acid solution. This method has proved to be very successful in acid soils.
In view of the high efficiency of the fluoride ion in dissolving phosphate, Bray (1945) recommended the use of this reagent together with HCl as an extractant (effectively removed sorbed phosphate).
Al reacts with F and inactivates Al leaving P in solution. Use of NH4F will increase extractable P, or stabilize P (restricting Al from precipitating with P because of the solubility constants).
Mehlich II
0.20 NH4Cl, 0.2N CH3COOH, 0.015N NH4F and 0.012N HCl
(pH = 2.5)
The concentrations of HCl and NH4F used in Mehlich are half that used in Bray and Kurtz P-1. However this extracting solution also contains NH4Cl and acetic acid which probably buffer the solution (i.e., keeps its acidic strength for a longer period of time). Therefore, it can dissolve more of the P in apatite.
Mehlich III
0.2N CH3COOH, 0.015N NH4F, 0.25N NH4NO3, 0.13N HNO3, 0.001M EDTA (pH = 2.4)
Designed to be applicable across a wide range of soil properties ranging in reaction from acid to basic. Can also be used for exchangeable cations (Ca and Mg). Because this extractant is so acid, there is some concern that the soil can be dissolved, increasing exchangeable amounts.
Olsen
0.5N NaHCO3 (pH = 8.5)
This extracting solution is used to extract phosphorus in calcareous soils. It will theoretically extract the phosphorus available to plants in high pH soils. This extractant decreases the concentration of Ca in solution by causing precipitation of Ca as CaCO3; as a result, the concentration of P in solution increases.
Essentially, increase the activity of CO3 in solution which reacts with Ca, and CaCO3 precipitates.
Nelson et al. (1953) (Mehlich I and or "Double Acid")
0.05N HCl and 0.025N H2SO4 (pH<2.0)
Found to be effective in high P-fixing
soils of
Extractable P discussion:
The pH of the extracting solution is an indicator of what forms of P will be extracted. However, this should be used with caution as the shaking time is important in terms of reaching an equilibrium.
Susuki et al. (1963) noted that 0.1N HCl extractable P was positively correlated with Ca-P.
NaHCO3
was negatively correlated with Ca-P on 17
What would happen if Bray P-1 was used on a calcareous soil?
The lime in the calcareous soil would neutralize the acidity in the extracting solution thus decreasing its ability to extract the Fe and Al-P forms which would be available at that soils pH.
Calibrations
for the Bray-Kurtz P-1, Mehlich III and Olsen soil tests (Tisdale, Nelson,
Beaton and Havlin, 1993)
____________________________________________________________________________
P sufficiency level Bray-Kurtz P-1 Mehlich III Olsen Fertilizer P
Recommendation
____________________________________________________________________________
lb
P2O5/ac kg P/ha
Very low <5 <7 <3 50 25
Low 6-12 8-14 4-7 30 15
Medium 13-25 15-28 8-11 15 8
High >25 >28 >12 0 0
____________________________________________________________________________
Analysis for total P in soils was abandoned in the early 1900's as scientists recognized that this analysis was not correlated with plant availability. For this reason various strengths of extracting solutions were evaluated for specific soils at selected soil pH that mirrored what the plant would find in soil solution. All of these are indices that determine orthophosphate concentrations (from the dissolution of precipitated forms). Attempts to correlate extractable P (x - procedure) with total P will result in meaningless information. Total P (strong acid digest) will in essence dissolve P forms that will not be available at that soils specific pH.
Bray and Nye:
K applications on soils with high K by mass action displace Al+++ which complexes with P inducing a net P deficiency (pH < 6.0)
P and Zinc
Zinc deficiencies attributed to the immobilization of zinc owing to the increase in the concentrations of P in the roots above the threshold values.
Depression of zinc concentrations in plant tissue by P (interaction occurred in the plant and not in the soil).
Source of N by P
NO3- uptake (increase pH)
NH4+ uptake (decrease pH)
Light is considered to be a stream of particles. The discrete particles or units of energy are
called photons or quanta. A photon of
blue light contains much more energy than a photon of red light.
Interaction of light with matter
1nm = 1mu (millimicron) = 10A (angstrom) = 10-7 cm
The interaction of radiation with matter may result in the absorption of incident radiation, emission of fluorescence or phosphorescence, scattering into new directions, rotation of the plane of polarization, or other changes. Each of these interactions can provide useful information about the nature of the same in which they occur (Tinoco et al. 1978).
Color is characteristic of the spectrum (in the visible region) of light transmitted by the substance when white light (or sunlight) shines through it, or when light is reflected from it.
_____________________________________________________
<0.01 Gamma (non
particulate photons)
0.01-10 X-Ray (photons)
10-380 Ultraviolet
Wavelength
absorbed, nm Absorbed Color Transmitted Color (Complement)
380-450 Violet Yellow-green
450-495 Blue Yellow
495-570 Green Violet
570-590 Yellow Blue
590-620
620-750 Red Blue-green
________________________________________________________________
750-1x106 Infrared
1x106-1x1011 Micro
and short
radio
waves
>1x1011 Radio,
FM TV
________________________________________________________________
Wavelength: distance of one complete cycle
Frequency: the number of cycles passing a fixed point per unit time

l = c/v
l = wavelength in cm
v = frequency in sec-1 or hertz (Hz)
c = velocity of light in a vacuum (3x1010 cm/sec)
Electromagnetic radiation possesses a certain amount of energy. The energy of a unit of radiation, called the photon is related to the frequency by E = hv = hc/l
where E is the energy of the photon in ergs
h is Planck’s constant 6.62 x 10-27 erg-sec
The shorter the wavelength or the greater the frequency, the greater the energy. Energy of a single photon (E) is proportional to its frequency (v) or inversely proportional to its wavelength.
If a molecule absorbs radiation, it is raised to a higher energy level, with the increase in energy being equal to the energy of the absorbed radiation (hl).
The relative energy levels of the three transition processes are in the order electronic > vibrational > rotational.
If the electromagnetic force results in a change in the arrangement of the electrons in a molecule, we say that a transition to a new electronic state has occurred. The absorbed photon results in the excitation of the molecule from its normal or ground state, G, to a higher energy or excited electronic state, E. The excited electronic state has a rearranged electron distribution.
When considering absorbing substances that are either liquids, solids or gases, each will have a characteristic transmission of light. Suppose that light of intensity Io is incident from the left, propagates along the x direction and exits from the right with decreased intensity It. At any point x within the sample, it has intensity I, which will decrease smoothly from left to right.

If the sample is homogeneous, the fractional decrease in the light intensity is the same across a small interval dx, regardless of the value of x. The decrease for a solution depends linearly on the concentration of the absorbing substance.
· Not all molecules can absorb in the infrared region
· The wavelength of absorption is a measure of the energy required for the transition
· Each molecule will have a complete absorption spectrum unique to that molecule, so a 'fingerprint' of the molecule is obtained
Soil Testing versus Non-destructive Sensor Based VRT
Soil
Testing Sensor
Based VRT
low
resolution high
resolution
Chemistry-Site
specific Site
specific
Reliable
and tested untested
Years of
correlation/calibration new
technology
Economical high
potential of being economical
Crop
specific untested
Variety
specific untested
Management
specific untested
row spacing/tillage
Nutrient
interactions untested
NA weed
recognition
NA time
of day
NA shadow/clouds
NA direction
of travel

Experimental
Design/Soil Testing and Field Variability
Replication gradients: Do slopes (up and down or side to side) in fields adequately represent which direction a particular nutrient will increase or decrease? Are Blocks actually needed?
Number of Replications: If plot size remains large and greater than the field element size, increasing the number of replications will unlikely lead to increased power for detecting differences between treatments.
Plot Size: Because field variability has been demonstrated to be somewhere around 9 square feet, field experiments as we now know them must change. Common plot sizes are between 250 to 1000 square feet. Plant breeders have generally employed much smaller plot sizes and because of this, CV's from their work are generally smaller than that found in fertility/weed type trials.
Documented as essential element by
Broyer et al. (1954). Deficiencies are
rare, and appear to be limited to in-land regions that have not required K
fertilization.
Chlorine is absorbed as Cl-. Cl is mobile
in the soil and in plants.
In
Plants
Average concentration in plants ranges from 1
to 20 g kg-1 (0.1 to 2%), while the concentration required for
optimum growth ranges from 150-300 mg kg-1 (0.015-0.03%).
Cl functions in plants mainly as a
mobile anion in processes related to osmotic pressure regulation (stomatal
openings) and charge compensation (as a counter ion in cation transport).
General crop requirement is about
1 unit Cl for 10,000 units of dry matter produced, or about 2-3 kg ha-1. (
Critical deficiency concentration
for optimum growth is reported to range from 70 mg kg-1 in tomato to 1000 mg kg-1 in kiwi.
Recent studies suggest the critical toxicity concentrations range from
3-5 g kg-1 in sensitive
plant species and 20-40 g kg-1 in tolerant plant species.
Deficiency symptoms include
reduced root growth, wilting and curling of leaves and leaflets, bronzing and
chlorosis similar to that for Mn deficiency.
In
Soils
Critical soil test level of 43.5
kg ha-1 of Cl in the upper 60 cm suggested by Fixen for
identifying responsive soils.
Most common fertilizer source of
Cl is muriate of potash (0-0-62), KCl.
In
plants
Critical deficiency concentration
ranges from 5-10 ppm in
monocotyledons to 50-70 ppm in dicotyledons, to as high as 100 ppm in
latex producing plants such as dandelion.
The critical toxicity
concentration is not much higher than the deficiency concentration. In corn it is about 100 ppm and cucumbers 400
ppm.
The main function of boron is in
cell growth and formation. The action
appears to be in binding sugars together in cis-diol ester linkages, resulting
in B being strongly complexed in cell walls.
An example of this is shown in the following generalized reactions.

Boron is immobile in plants.
In
soils
Boron in aqueous solution is
present mainly as undissociated boric acid, B(OH)3. It dissociates according to the equation:
B(OH)3 + HOH ——>
B(OH)-4 + H+ Keq = 10-9.2
[B(OH)-4] [H+]/[B(OH)3 ] = 10-9.2
and, rearranging we have
[B(OH)-4] /[B(OH)3] = 10-9.2/[H+]
taking the log of both sides, results in
log [B(OH)-4] /[B(OH)3] = -9.2 -log [H+],
or
log [B(OH)-4] /[B(OH)3] = -9.2 + pH
and at pH 7.2, log [B(OH)-4] /[B(OH)3 ] = -2,
so there is 100 times less B(OH)-4 than [B(OH)3](the ratio is .01),
verifying that B(OH)3 is the
predominate B species in the soil solution of agricultural soils.
Hence, unlike all other nutrients plants obtain from the soil, B is
apparently taken up as the uncharged B(OH)3.
Boron is mobile in soils.
In
plants
Found in plants primarily as the
oxyanion (oxidation state VI), but also as Mo (V) and (IV).
Mo is absorbed as MoO4=, since it is the dominant species above pH 4.5 (see
Fig. 10.1 below, taken from Micronutrients in Agriculture)

Figure 10.1. Relationship of molybdate ion species to pH.
Mo functions in electron transfer
in plants, primarily in nitrate reductase (see Fig. 10.2 from Micronutrients in
Agriculture) in non-legumes and nitrogenase (see Fig. 10.3 from Micronutrients
in Agriculture) in legumes.
In each case N reduction is
involved. Plants supplied with NH4+ have a much lower demand for


Figure 10.2. Structural model of the
nitrate reductase with its two subunits. Each subunit contains three prosthetic
groups: FAD, heme-Fe, and Mo-pterin.
(Based on Campbell, 1988)
Figure 10.3. Model of the stepwise N2 reduction by the Mo-containing nitrogenase.
The critical deficiency level
ranges from 0.1 to 1 ppm in leaves, whereas critical toxicity concentrations
range from 100 to 1,000 ppm.
Mo is readily translocated and
deficiency symptoms are normally found in the oldest leaves. For legumes the symptoms are like that for N
deficiency. In non-legumes the condition
of “whip tail”, where leaf blades are reduced and irregularly formed is common
together with interveinal mottling, marginal chlorosis, and accumulation of NO3.
In
Soils
The normal concentration of Mo is
quite low, ranging from about 1 to 10 ppm.
Deficiencies are uncommon, but are
more likely in acid than alkaline soils apparently because MoO4= is strongly adsorbed to iron oxide surfaces in acid
soils, either as a result of chemical bonding or simple anion exchange associated
with pH dependent charges in acid soils..
Liming these acid soils increases the availability of Mo and is a common
procedure for correcting Mo deficiency.

Bonding mechanisms

Exchange mechanisms
In
Plants
The deficiency concentration of Fe
in mature plant tissue is about 50 ppm.
Total Fe may be much higher than this level, even in Fe-chlorotic plants
because Fe in the plant is not always all metabolically active. HCl extractable Fe is sometimes assumed to be
metabolically active and a better guide to plant sufficiency. Fe in plants is found in the Fe+++ state, any Fe++ is present only as a transitory state (free Fe++ is phytotoxic).
Fe functions as a co-enzyme, in important
electron transfer enzymes, and the formation and component of enzymes that are
precursors to chlorophyll. The two
important categories of enzymes are the Fe-S proteins and the heme proteins.
Heme proteins are characterized by
a tetrapyrrole ring structure that has Fe as the centrally coordinated
metal. Fe is involved as a co-factor in the
synthesis of protoporphyrin, which is the precursor to both heme and
chlorophyll as depicted in Fig. 10.4.
The Fe-S proteins are formed when
Fe is coordinated to the thiol
group of cysteine, or inorganic S,
or both (see illustration below).

Figure 10.4. Role of Fe in biosynthesis of heme coenzymes and chlorophyll.
The best known Fe-S protein is
ferredoxin, important in both
nitrate reductase and nitrogenase.
Other Fe-S proteins have important functions in the citric acid cycle,
respiration, SO4 and SO3 reduction, and chlorophyll (see
Fig. 10.5).


Because Fe is strongly bound it is not easily
translocated and should be considered immobile in plants. The characteristic deficiency symptoms are
interveinal chlorosis in the new leaves of growing plants.
Figure 10.5. Role of Fe and other micronutrients in the photosynthetic electron transport chain. PS=photosystem (PS I, PS, II); S = water-splitting enzyme; g~4 = non-heme Fe-S group; Z = tyrosine residue-containing electron donor to P 680; P 680 = primary electron donor of PS I; Ph = primary electron acceptor pheophytin; QA = quinone-Fe complex; PQ = plastoquinone; Cyt = cytochrome; PC = plastocyanin; and X, B, and A = Fe4S4 proteins. Schematically drawn as Z scheme. (Based on Terry and Abadia, 1986; Rutherford, 1989.)
Iron
in soil
Soils contain about 1 to 5% iron,
which is many fold more than that
required for plants, however, in
aerobic environments Fe is mainly present in the Fe+++ oxidation state as iron oxide (written as either Fe2O3. nH2O or Fe(OH)3 ) which is very insoluble. The amount of Fe+++ in aqueous solution is governed by
Fe(OH)3 <_____> Fe+++ + 3(OH) , for which the equilibrium condition
is expressed in molar concentrations as
(Fe+++)(OH)3/ Fe(OH)3 = 10-39.4 (1)
Since Fe(OH)3 is a solid, it has an activity of unity (1) and the
equation becomes
(Fe+++) (OH)3 = 10-39.4 (2)
and the value 10-39.4, instead of being called the equilibrium constant (Keq),
is called the solubility product constant (Ksp). The concentration
of Fe+++ in solution is given by
Fe+++ = 10-39.4 / (OH)3 (3)
and at pH = 7.0,
Fe+++ = 10-39.4 / (10-7)3 ; =10-39.4 / 10-21 ; = 10-18.4 moles/liter.
Since the atomic weight of Fe is
55.85, the concentration in ppm would be
55.85 g/mole x 1000 mg/g x 10-18.4 moles/liter =
55.85 x 10-15.4 mg/liter; =
55.85 x 10-15.4 ppm
The plant’s dilemma: The concentration of Fe necessary to provide
plants a sufficient amount of Fe by passive uptake has been suggested to be
about 10-6 moles/liter.
At pH 7 the soil supply, as identified by equation (3) is 12 orders of
magnitude too small! Even at pH 5 the
difference between supply and requirement is still 6 orders of magnitude too
small (students should verify this by calculation).
Two things are obvious; (a) the
plant cannot get enough Fe by passive uptake from the soil solution, and (b)
there will be a 1000 fold decrease in supply of available Fe from Fe(OH)3
in the soil with each unit increase in soil pH.
Consequently, one should expect Fe deficiency to be most common in high
pH soils and least in acid soils. This
is in fact what is observed. But, how do
plants get enough Fe, and why are not all plants subject to Fe chlorosis when
grown in neutral and alkaline soils?
Part of the solution to the
plant’s dilemma of getting enough Fe from the soil is found in chemical
reactions called metal chelation. This
is the process whereby metals are bound in ring-like structures of organic
compounds. The more rings in the
structure that the metal is a part of, the stronger the metal is bound. The chemical forces involved are mainly
coordinate bonds where the metal acts as a Lewis acid (electron acceptor) and
the chelating material has functional groups (sometimes called ligands), like
amino, hydroxyl, and phenolic groups that act as Lewis bases (electron
donor). The transition metals seek to
fill the d orbital to attain the electron configuration of the inert gas of
that period, krypton. Heme and chlorophyll
are examples of natural chelates that hold Fe and Mg as a centrally coordinated
atom.

As an example of chelates, consider the common
synthetic chelate EDTA. EDTA stands for the chemical compound
ethylenediaminetetraacetic acid.

Note in the Fe-EDTA complex there are five rings
formed with Fe, and that the complex
has a single negative charge. As a
result, the complex is mobile in the soil and so is the Fe it is carrying. Two other common synthetic chelates are DTPA
(commonly used in micronutrient metal soil test extraction procedures) and
EDDHA (a commercial chelate for supplying Fe in calcareous soils).
Figure 10.6. Sequestrene 330
Fe (DTPA) is monosodium hydrogen ferric diethylenetriamine pentaacetate, which
has a molecular weight
of 468.

Figure 10.7. Chel 138 HFe
(EDDHA) is hydrogen ferric ethylene bis (alpha-imino-2-hydroxy-phenyl-acetic
acid), which has a molecular weight of 413.
Two important natural chelating
compounds in plants are citrate and hydroxamate. Citrate is important as a carrier for the
micronutrient metals Cu, Zn, Fe, and Mn.
Hydroxamate is a siderophore (produced by micoorganisms) believed
responsible for complexing Fe in calcareous soils and increasing its
availability to plants.
The reaction of chelates with
metals to form soluble metal chelates is given by the general equilibrium
reaction
M
+ L = ML (4)
Where M refers to the metal
concentration, L the chelate (or Ligands) concentration, and ML the
concentration of metal chelate. At
equilibrium the relative amounts of each present are described in relation to
the equilibrium constant as
ML
/ (M) (L) = Keq (5)
Since the equilibrium condition
strongly favors the reaction to the right (equation 4), Keq is
called the formation constant Kf.
The formation constants for citrate and hydroxamate are 1012.2
and 1032, respectively.
The benefit of chelates for
improving Fe availability can be demonstrated by considering just the reactions
involved in chelates complexing Fe from Fe(OH)3. If the reactions are considered
simultaneously they can be written as follows for an equilibrium situation
where a weak chelate such as citrate is present.
Log10 K
Fe(OH)3 ————> Fe+++ + 3(
Fe+++ + L ————> FeL 12.2
3(OH)
+ 3H+ ——————> 3H2O 42
Fe(OH)3 + L + 3H+ ————>
FeL 14.8
Fe(OH)3 + L + 3H+ ————>
FeL 1014.8 (6)
Equation (6) was obtained by
summing the equations (canceling
components that appear as both reactant and product of the reactions)
and the log10 of the solubility and formation constants. The concentration of products and reactants
can be expressed for the general reaction in terms of the equilibrium constant
as
(FeL) / (L) (H+)3 = 1014.8
or in terms of (FeL) as
(FeL) = 1014.8 x (L) (H+)3
If the soil pH is 7 and the
concentration of citrate is 10-6 , then the concentration of FeL is
(FeL) = 1014.8 x (10-6)
(10-7)3
(FeL) = 10-12.2
Compared to the concentration of Fe+++ in solution from Fe(OH)3 dissolving,
which is 10-18.4, this is an improvement of 106.2 (10-12.2 / 10-18.4). In other words, the presence of even a weak
chelating agent like citrate has improved the availability of iron a million
fold!
One should be aware, that in the
case of a metal nutrient like Fe, the concentration of FeL in the soil solution
is mainly a function of the formation constant (Kf) and the
concentration of L since the other factors are constant. For example, consider Eq. (5)
ML / (M) (L) = Keq
This can be rewritten as
ML = Keq (M) (L)
Where M is Fe+++, and is a constant identified by the solution pH and Ksp for Fe(OH)3. Since Keq is also a constant, these can be combined into
one constant, to give
ML
= K (L)
(7)
Equation (7) identifies that any
condition that results in increasing the concentration of L for complexing or
chelating Fe will increase the concentration of FeL and thus the availability
of Fe for the plant. The two most
obvious ways of increasing L will be by
(1) drying the soil so the water soluble L will become more concentrated
and, (2) producing more L in the soil solution.
In fact, a common observation is that Fe chlorosis is lessened in
susceptible plants when there are definite drying cycles as opposed to
continuously moist soil. Also, Fe
chlorosis can often be lessened by
incorporating large amounts of decayed or decaying organic matter to the
soil which will directly provide more L.
Plant absorption of soil Fe
(Dicotyledons)
Until recently, the mechanisms
responsible for allowing some species of plants to grow well in calcareous soils
while others commonly exhibited iron chlorosis were not well understood. The observation that some dicots were “iron
efficient” while others, even varieties within a species, were “iron
inefficient” is explained by an adaptive response mechanism inherent in iron
efficient plants. Characteristics of
this mechanism, which is activated when plants are in an Fe stress situation
are
1.
Enhanced
root-associated Fe+++ reduction
2.
Enhanced H+ efflux from roots
3.
Accumulation of
citrate in roots
4.
Increased root hair
development
5.
Increased
absorption of Fe
A description of the mechanism is
illustrated in Fig. 10.8.

Figure 10.8. Ferric-chelate reduction-based model depicting the various physiological processes thought to be involved in the reduction of Fe(III) at the root-cell plasma membrane, and the subsequent absorption of Fe(II) ions into the root-cell of dicot and nongraminaceous monocot plants. Central to this model is the inducible Fe(III) reductase (Ri) in the plasma membrane that is induced in response to Fe deficiency. A constitutive Fe(III) reductase (Rc) is hypothesized to function under Fe-sufficient conditions.
Grasses
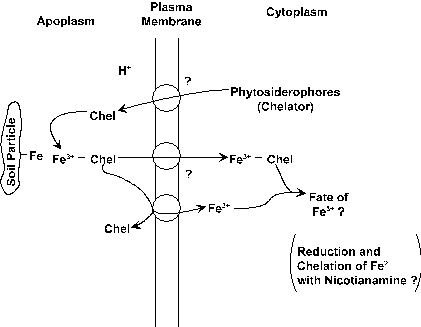
In grasses, Fe uptake is enhanced by a different
mechanism, one that relies on the plant production of phytosiderophores. Phytosiderophore is a term used to describe
plant produced chelates or complexing material that can increase the
availability of Fe+++. An
illustration of this mechanism is provided in Fig. 10.9.
Figure 10.9. Phytosiderophore-based model for Fe absorption in grasses.
In
plants
Manganese is absorbed as Mn++
The critical deficiency
concentration is about 10 - 15 ppm.
Deficiency symptoms include “gray speck”
in cereals, a condition that results when there is interveinal discoloration on
the middle-aged leaves.
Mn functions in electron transfer
processes and as a co-factor for some enzyme reactions. The most widely known function is probably
its involvement in the Hill reaction of photosynthesis, wherein there is a 4e- transfer that results in the splitting of water and
release of O2.
2H2O + 4e- —> 4H+ +O2
In
soils
Mn concentration in soils varies
widely (20 to 3,000 ppm) depending upon parent material and the degree of soil
weathering.
Mn is easily oxidized from the Mn++ to Mn+++ and Mn++++ , the Mn++ is not strongly chelated, while the Mn++++ may be strongly complexed. Oxides of the highest valence state are quite
insoluble, hence availability can be greatly affected by redox potential. Mn uptake is improved in some plants if they
undergo Fe stress and are able to respond by producing a reducing agent since
it will reduce both iron and manganese to more soluble oxidation states.
Deficiencies are most common in
highly weathered soils that have been recently limed.
In
plants
The critical deficiency
concentration is about 1 to 3 ppm.
Typical deficiency symptoms are
chlorosis (white tip), necrosis, and die-back in the youngest leaves.
Cu is absorbed as Cu++ and is relatively immobile in the plant.
Because it undergoes
oxidation-reduction reactions relatively easily, Cu is involved in electron
transfer and enzyme systems much like Fe, most notably the oxidase enzymes.
In
soils
Of the divalent cations, Cu++ is the most strongly complexed by organic
matter. Deficiencies are most common in
high organic (peat) soils because the Cu in the soil is bound too tightly for
plants to extract adequate amounts.
As much as 98% of all the Cu++ in the soil solution may be present as organic
complexes.
In
plants
The critical deficiency
concentration is from 15 to 30 ppm, higher if leaf P is above normal.
Zn has only one oxidation state as
an ion, Zn++. Zn++ is immobile in both the plant and soil.
Zn functions as an ion for
coupling enzymes and substrate. The most
common Zn containing enzyme is alcohol dehydrogenase.
Deficiencies are manifested by a shortening
of internodes to the extent it appears leaves are all emanating from the same
point on stems (condition is called “rosetting”). In corn, chlorotic bands appear along the
leaf midrib. Zn deficiency symptoms on
older leaves is mainly a result of P toxicity (retranslocation of P is
inhibited by Zn deficiency).
Deficiencies are common in pecans and corn, but have not been reported
for wheat even in very deficient soil.
In
soil
Total content ranges from 10 to 30
ppm
Availability is closely linked to soil pH and organic matter content.
1.
Broadcast
·
N (in zero
tillage on acid soils, not a good idea) - increased acidity.
·
Increased N needs
in zero tillage (1. immobilization, 2. leaching)
·
P (in zero
tillage - horizontal band)
2.
Band
·
Dual placement
Not
a good idea on acid soils (banding P and N) - increased acidity will bring Al+++ and Mn++ into solution.
Works
well in calcareous soils where increased acidity will increase micronutrient
availability.
Plant
needs for micronutrients can be satisfied with the localized band (synergistic
effect of placing nutrients within an area, results in increased root growth
within that zone: root probability).
Dual
placement in calcareous soils can be beneficial when anhydrous ammonia is used
as the N source and an ammonium form of N is taken up by the plant. Uptake of ammonium will result in a decreased
rhizosphere pH thus enhancing P availability
(H2PO4 : HPO4 ratios).

3.
Foliar
Foliar
applications have generally been used for micronutrients where a severe
deficiency warranted the expense of applying fertilizers via this method.
Topdress
applications of UAN, via center pivot systems
has become increasingly popular with time (apply the N when it is
needed).
Acid neutralized ammonia: Anhydrous ammonia injected into the
irrigation pipe followed by injections of H2SO4 to lower water pH. This method has not been used commercially,
because of the fear associated with handling large quantities of industrial
grade H2SO4. It does make
sense when considering that AA is 1/2 the price per unit N compared to UAN.
Aqueous ammonia: Anhydrous ammonia
bubbled into ditch irrigation systems without the use of H2SO4 (common in
irrigated regions of
Accumulated salts contain Na, Ca,
Mg and Cl, SO4, HCO3 and CO3. Na can be toxic to plants and acts as a
dispersing agent, reducing soil drainage (slick spots).
Problem is caused by the
dispersion of small size clay particles which plug soil water flow channels
(destroys soil structure).
Fine textured soils with
montmorillonitic clays may disperse when 15% of the exchange complex is
dominated by Na.
Tropical soils high in Fe and Al
oxides may require 40% Na saturation before dispersion is a problem.
Saline (Arid and semi-arid
regions): Function of poor drainage
accompanied by high evaporation rates (salts accumulate at the surface). Saline soils are generally 'man-made problem
soils' where fertilizers have been applied and where poor drainage and or where
poor quality (high salt content) water is used for irrigation. Over 2 %/yr of the arable land present in the
world today is taken out of production due to salinity/sodicity problems
Sources of salt:
a.
natural
weathering (parent materials)
b.
fertilizer
c.
irrigation water
d.
fossil salts
(gypsiferous sediments)
e.
rain (near the
ocean)
Measurement:
EC (electrical conductivity) is
the inverse of Resistance (ohms)
*note: water quality is measured
in resistance, high purity = high resistance
a.
Salinity is
conventionally measured on aqueous extracts of saturated soil pastes
b.
Crop tolerance to
salinity is often related to the electrical conductivity or total electrolyte
concentration of the saturation extract
Reagent: Sodium hexametaphosphate
(NaPO3)6 0.1%
(added
to prevent precipitation of CaCO3)
Saturation Extract: 200 to 400 g
of soil (do not oven dry)
1.
Weigh soil +
container
2.
Add distilled
water until nearly saturated
3.
Mix and allow to
stand overnight
4.
Weigh container +
contents (record increase in weight)
5.
Transfer to a
Buchner filter funnel, apply vacuum and collect filtrate (if turbid, re-filter)
6.
Add 1 drop of
0.1% (NaPO3)6 for each 25 ml
of extract
Major solutes of interest: Ca, Mg,
Na, K, CO3, HCO3 SO4, Cl, NO3 and H3BO3
Ability of the soil solution to
conduct electrical current
new units: dS/m (decisiemens per meter)
old units: mmho/cm (millimhos per cm)
1 mmho/cm = 1dS/m
Plants must overcome solution
osmotic potential to absorb water.
increased EC - increased OP -->
results in decreased H2O availability
OP = EC(-0.36)
Reclamation:
1. Saline
a.
wash with water
(low salt content)
b.
must be leached
below the root zone
2. Saline-Sodic
a.
replace Na on the
exchange complex with Ca by adding gypsum
b.
wash with water
3. Sodic
a.
resolve sodic
problem first (apply CaSO4 to exchange for Na)
b.
wash with water
4.

Reclamation:
All require long periods of time
to reclaim and all have drainage problems.
In each case, addition of organic matter (incorporation) will assist
with drainage.
Early estimates of the relative
sodium contents of water were based solely on their percent sodium
content. Waters with high Na may produce
relatively low exchangeable Na levels in soils if the total cation
concentration is high (Bohn, p 225).
SAR (sodium adsorption ratio) was proposed
to characterize the relative Na status of irrigation waters and soil solutions.
SAR = [Na+] / ([Ca + Mg]/2) 1/2
where all concentrations are in
meq/liter.
The Ca + Mg is divided by two
because most ion exchange equations express concentrations as moles/liter or
mmoles/liter rather than meq/liter.
Allows us to gain information
about the exchangeable cations without actually taking an actual measurement.
Amounts adsorbed are proportional
to the amounts in soil solution (Donan Equilibrium Theory).
(measurement of soil solution and
not exchange)
Linear regression of yield on the
mean of all treatments (varieties) for each site and season. Original work employed a logarithmic scale.
Objective
(Plant Breeding)
a.
Mean yield of all
varieties provided a quantitative grading of environments.
b.
Varieties
specifically adapted to good or poor environments were identified.
Objective (Soil Fertility)
a.
To assess treatment
response as a function of environment and to detect the benefits of using these
analyses to complement conventional analysis of variance.
Eberhart and Russell, 1966
Yij = ui +
Bi Ij +
eij
Yij = variety
mean of the ith variety and jth environment
ui = ith variety
mean over all environments
Bi = regression
coefficient that measures the response of the ith variety to varying environments
eij = deviations
from regression of the ith variety at the jth environment
Ij = environmental
index
Defined a stable genotype as one
having deviations from regression = 0 and a slope of 1.0
Analysis of Variance: (Over
Locations)
10 locations
10 varieties
3 reps
Source of Variation df
_____________________________________________________
Total 299
Environment (e-1) 9
Rep(Environment) (r-1(e)) 20 (error A)
Genotype (g-1) 9
Genotype * Environment (g-1)(e-1) 81
Residual Error 180
_____________________________________________________
df
- degrees of freedom
G*E
interaction
Stability analysis is essentially
a method of partitioning the G*E interaction term assuming that environment
could be quantified. In general,
environment means in stability analysis are assumed to be a function of
temporal variability and that genotype response was a direct function of that
variable which influenced yield potential.
This has most generally been attributed to high or low rainfall.
A major purpose of long-term
fertility trials is to provide a measure of the effect of environment over time
on the consistency of treatment effects.
Assessing year X trt interactions in long-term fertility experiments is
an issue when more than two or three years of data are present. Interpretation of year X treatment
interactions using analysis of variance is difficult due to the number of
factors affecting environment.
Initial use of regression to
assess yield stability of genotypes across a wide range of environments was
originally presented by Yates and Cochran (1938) and later followed by Finlay
and Wilkinson (1963) and Eberhart and Russell (1966). The technique is useful in relating a
measurement of environment which is usually the mean yield across all genotypes
for each environment to performance of different genotypes tested. Eberhart and Russell, (1966) characterize a
stable genotype as having a linear regression coefficient of one and deviations
from regression equal to zero.
The extrapolation of some of these
concepts to characterize stability of agronomic treatments instead of genotypes
seems to be a practical application in separating the response of treatments as
a function of environment over time.
This assumes that the lack of consistency of treatment effects over time
(a treatment X year interaction) can be interpreted as a linear function of the
environment mean on the mean yield for a given treatment. Hildebrand (1984), stated that it is visually
possible to compare treatments and to generalize these equation sets for
various kinds of management practices, and further states that the environment
mean measures treatment response to good or poor environments regardless of the
reasons these environments were good or bad.
Stability Analysis for
single-site-long-term experiments:
Analysis of Variance: (
10 years
10 treatments (N, P, K fertilization,
Herbicide trt, etc)
3 reps
Source
of Variation df
_____________________________________________________
Total 299
Replication 2
Treatment 9
Replication*Treatment 18 (error A)
Year 9
Year*Treatment 81
Residual Error 180
_____________________________________________________
df
- degrees of freedom (weak test for
treatment, 18 df)
Results:
K supply in a stress environment
showed increases in yield. Why? This observation was the trt*environment
interaction.
Anhydrous ammonia superior in
stress environments. Why? NH4 supply - immediate glutamine formation.
Stability Analysis: discussion
It is conceivably difficult to
predict the environment mean since variety, rainfall, weed pressure and disease
are variable from year to year. In an
additive linear model like those used in conventional analysis of variance, the
mathematical sums of squares accounted by year, treatment and year X treatment
effects are removed from the random variation (residual error), yet year and
year X treatment effects are seldom interpreted from a biological point of
view. Limited biological interpretation
of the lack of consistency of treatment effects over years (year X treatment
interaction) decreases the value of conventional analysis in identifying
treatment advantages as a function of environment.
The use of stability analysis
implies that treatment is actually a linear function of temporal variability
which would complement some of the limitations encountered in conventional
analysis of variance. Hildebrand (1984)
states that stability analysis explicitly incorporates variation in farmer
management as well as in soils and climate to help agronomists evaluate
responses to treatments and partition farmers into recommendation domains. In depth analysis of year X treatment
interactions suggests that the researcher should view changed treatment
response within the specific environment in which the treatment differences
were observed. When considering 2 or 3
years of data, the year X treatment interaction can be easily separated into
discrete components using specific comparisons by means of non‑orthogonal
contrasts. However, it is unlikely that
biological interpretation of the year X treatment interaction will be achieved
when faced with 10 or more years of data using conventional analysis. Alternatively, stability analysis is in
effect somewhat restricted to long-term experiments and/or multilocation
experiments since adequate degrees of freedom are needed to obtain meaningful
regressions.

In general, differences in environment means for
single-site long-term experiments can largely be attributed to moisture
availability. This observation could
assist in identifying potential differences between fertilizer treatments in
either reduced or oxidized environments.
Work by Olsen (1986) discusses the differences between ammonium and
nitrate nutrition as related to energy use and factors which affect
availability.
It is of some concern as to how
residual treatment effects influence yield in succeeding cycles. If treatment response was a function of a
particular environment, then it seems reasonable that detection of residual
treatment effects will be affected by the previous environment. However, plots of grain yield by year did not
reveal any evident patterns of residual treatment effects. Furthermore, in stability analysis the
environment mean while random, is in effect ordered in succession thus
confounding any detection of residual treatment effects if they existed. Nonetheless, conventional split plot in time
analysis of variance models are no better in this regard since residual effects
are also not evaluated. It should also
be mentioned that stability analysis over locations versus one-site long-term
experiments presents a problem of correlated yield results over time or
autocorrelations in the data for the latter mentioned example.
When year X treatment interactions
are detected in the conventional analysis of variance model, ensuing stability analysis
provides a simple method of determining whether or not this interaction is a
function of environment. Although this
can also be achieved by partitioning the degrees of freedom in the year X
treatment interaction from the analysis of variance model, stability analysis
may provide a more direct method of assessing temporal variability in long-term
experiments.
Recommendation strategies could
possibly be refined by the added use of stability analysis when assessing
agronomic treatment response over time.
As issues of sustainability become increasingly important, stability
analysis and relative stability may assist in our understanding of yield as a
function of environment as well as identifying areas that warrant further
investigation.
K° = equilibrium constant
expressed in terms of activities
K°= (HL)/(H) (L)
What does K° mean?
·
log K°, high
positive number (dissociation will take place)
·
log K°, high
negative number (low solubility - slow dissociation)
10 -39.4 = (Fe)
(OH)3/ Fe(OH)3 indicates that
Fe will stay in this form Fe(OH)3
Example:
Activity of Al+++ (Xn) limited or controlled by gibbsite (Y)
Al(OH)3 + 3 H+ ——> Al+++ + 3 H2O Log K° = 8.04 (equilibrium activity constant)
gibbsite
Gibbsite is the most abundant free
hydroxide of Al in soils and occurs in large amounts in highly weathered soils
(Bohn, p. 89)
Al+++/ (H+)
3 = 10 8.04
Log Al+++ = 8.04 - 3pH
pH - 1/3 pAl = 2.68
-Log(H+) - 1/3 log(Al) = 2.68
Redox Relationships
reduction: gain electrons
oxidation: lose electrons
H atom atomic wt. = 1.007826
H ion (proton) = 1.007277
electron = 0.000549
Effect of redox on the
stability of Fe and Al phosphates:
When soils are flooded, we can
increase P availability in acid soils. The
pH of a reduced soil generally rises toward neutral (7.0) which increases the
solubility of Fe and Al phosphates. As
pe + pH drops below 8.34 (depending on which iron oxides control iron and which
minerals control Al+++) strengite and variscite convert to vivianite. Rice plants (grown under reduced conditions)
are able to obtain sufficient P in the presence of vivianite because in the
immediate vicinity of the root, redox is higher than that of the bulk soil because
O2 is supplied through the stem to the
roots. This is an example of how plants
absorb P where vivianite suppresses the solubility of P in the bulk soil to
very low levels (Lindsay, p 179).
Oxidation reduction reactions
in soils
redox potential (p3) is expressed as
(-log of electron activity) which is consistent with pH = - log(H+)
1.
Most soil systems consist of aqueous environments in which the
dissociation of water H2(g) or O2(g) impose redox limits
on soils.
K° = (H2(g))1/2/(H+) (e-)
log K° = 1/2 log H2(g) - log(H+) - log (e-)
The equilibrium constant for this
reaction is defined as unity (Log K° = 0) for standard state conditions in
which (H+) activity = 1 mole/l and H2(g)
is the partial pressure of H2(g) at 1 atmosphere.
since log K° = 0
pe + pH = 1/2 log H2(g)
therefore when H2(g) = 1 atm, pe + pH = 0
This represents
* most reduced equilibrium
conditions expected for natural aqueous environments.
On the oxidized side, redox limit
of aqueous systems is given by the reaction
H+ + e- + 1/2 O2(g) ——> 1/2 H2O
Equilibrium expression for this
reaction is K°=(H2O)½(H+)(e-)(O2(g))¼
The value of K° can be calculated
from the standard free energies of formation (Appendix, Lindsay, 1979) and is
equal to 10 20.78. In dilute
aqueous systems, the activity of water is very near unity, so the equilibrium
expression in log form becomes
-log(H+) - log(e-) - ¼ log O2(g)
= 20.78
pe + pH = 20.78 + ¼ log O2(g)
Therefore, when O2(g) is 1 atm, pe + pH = 20.78 which corresponds to most
oxidized equilibrium conditions expected in natural aqueous environments. The parameter pe + pH provides a single term
expression for defining redox status of aqueous systems. (range = 0 on reduced
side (1 atm H2) to 20.78 on the oxidized side (1 atm O2))
pH expressed - log (H+) activity
pe denotes - log (e-) activity
Activity of Al+++ is at equilibrium with various Al minerals (gibbsite,
etc) and is pH dependent (decreasing 1000x for each unit increase in pH).
When Al+++ is controlled by Al(OH)3 amorphous rather than gibbsite, the activity
of Al+++ is 10 9.66/10
8.04 = 10 1.62 or 42 times higher.
1.62(10x) = 41.68
Activity of Al+++ in soils is often below that of gibbsite due to the
presence of various aluminosilicates.
Because silicon is removed from
soils more rapidly than Al, weathering causes the eventual disappearance of
aluminosilicates. The Fe and Al that are
released generally precipitate as oxides and hydroxides (e.g., gibbsite which
is present in highly weathered soils).
In aqueous
solutions Al+++ does not remain as a free ion. It is normally surrounded by six molecules of
water (Al(H2O)6).
As pH increases, protons are
removed
Phosphorus
The figure on page 112 shows the relative
fractions of different orthophosphoric acid species as a function of pH. The formation constant (log K°) relating two
species is numerically equal to the pH at which the reacting species have equal
activities.
HPO4= + H+ ——> H2PO4- log
K° = 7.2
(H2PO4-)/(HPO4=)(H+)
= 10 7.2
log (H2PO4-)/(HPO4=) = log
K° - pH = 7.2 - pH
When pH = log K° the activity ratio of the reacting species is unity. A decrease in pH of one unit increases the ratio H2PO4-/HPO4= by a factor of 10.
Some Rules of Thumb for Predicting the Outcome of Simple Inorganic Chemical Reactions Related to Soil Fertility
For the general reaction:
An+ + Bm- ç=è AmBn (1)
Whether the reactant ions A and B
combine to form a compound (usually a solid) may generally be predicted by the
size of electrical charge in the ionic form.
Generally, the higher the charge of either the cation or anion, the
greater is the tendency for the compound or solid to be formed. When the solid is easily formed, only small
concentrations of the reactants are necessary for the reaction to take place. Because of this, the compound or solid that
forms is also quite insoluble (it will not easily dissolve in water), or it
does not easily break apart (reaction to the left). Conversely, if the cation and anion are both
single charged, then the compound (solid) is not as easily formed, and if it
does form, it is quite soluble. Here are
some examples:
1.
Single charged ions forming soluble compounds.
Na+ + Cl- ç=è NaCl (2)
We all have experienced that NaCl,
common table salt, is very soluble and easily dissolves in water. Once dissolved, the solid NaCl does not
reform until the ions, Na+ + Cl-, are present in high concentration. This happens when water is lost from the
solution by evaporation and the solid finally reforms as NaCl precipitate.
2. Multiple charged ions forming
insoluble compounds
When iron reacts with oxygen a
very insoluble solid, rust or iron oxide, is formed. The reaction can be expressed as
2 Fe3+ + 3 O= ç=è Fe2O3 (rust) (3)
With regard to solubility of inorganic
compounds, we may expect the following:
I. When
both the cation and anion are single charged, the resulting compound is usually
very soluble. Examples are compounds
formed from the cations H+, NH4+, Na+,
K+ and the anions OH-,
Cl-, NO3-, H2PO4,
and HCO3- (bicarbonate).
Also, when the cation reacts with
Except
for H+ and
a.
the divalent
cations Mg2+, Ca2+,
Mn2+, Fe2+, Cu2+,
Zn2+.
b.
the divalent
anions SO4=, CO3=
(carbonate), HPO4=, and MoO4=
c.
the trivalent
cation Fe3+
d.
the trivalent
anion PO43-
Accordingly,
when either of the monovalent anions Cl-, NO3- react with any of the cations Mg2+,
Ca2+, Mn2+, Fe2+,
Cu2+, Zn2+, or Fe3+,
the solids are all quite soluble.
Similarly, when any of the monovalent cations NH4+, Na+, or K+ reacts with any of the multicharged anions SO4=, CO3=, HPO4=, MoO4=, or PO43-, the solids are all quite soluble.
II. If
both the cation and anion are divalent, the resulting compound will be only
sparingly soluble. An example is gypsum
(CaSO4. 2H2O).
III. If one of the ions is divalent and the other is
trivalent, the compound will be moderately
insoluble. An example is
tricalcium phosphate, Ca3(PO4)2.
IV. If
both the anion and cation are trivalent, the compound is very insoluble. An example is iron (ferric) phosphate, FePO4.
A summary of these general rules
is illustrated in the following diagrams.
![]()
![]()
![]() M+++ M++ M+ A- A-- A---
M+++ M++ M+ A- A-- A---
![]()
A. All compounds with a monovalent
ion are soluble.
M+++ M++ M+ A- A-- A---
![]()
B. Compounds with both ions
divalent are sparingly soluble.
M+++ M++ M+ A- A-- A---
![]()
C. Compounds with one divalent ion
and one trivalent ion are moderately insoluble.
M+++ M++ M++ A- A-- A---
![]()
D. Compounds with both ions trivalent
are very insoluble
Alexander, M.A., 1977. Introduction to soil microbiology. 2nd
Edition. John Wiley & Sons, Inc.
Allison, Franklin E. 1966. The fate of nitrogen applied to soils. Adv. Agron. 18:219-258.
Altom, W., J.L. Rogers, W.R. Raun, G.V. Johnson and S.L. Taylor. 1996. Long-term rye-wheat-ryegrass forage yields as affected by rate and date of N application. J. of Prod. Agric. 9:510-516.
Aulakh, M.S., D.A. Rennie And E.A. Paul. 1984. The influence of plant residues on denitrification rates in conventional and zero tilled soils. Soil Sci. Soc. Am. J. 48:790-794.
Aylmore, L.A.G., Kavim Meshanul, and J.P. Quirk. 1967. Adsorption and desorption of sulphate ions by soil constituents. Soil Sci. 103:10-15.
Balba, Monem and Roger H. Bray. The application of the miterscherlich equation for the calculation of plant composition due to fertilizer increments. Soil Sci. Soc. Am. Proc. 20:515-518.
Barreto, H.J., and R.L. Westerman. 1985. YIELDFIT, Determining
maximum economic fertilization rates using Mitscherlich, quadratic and square
root functions. Comp. Software Series CSS-11.
Bear, F.E., A.L. Prince and J.L. Malcolm. 1945. Potassium needs
of
Blevins, R.L., M.S. Smith, G.W. Thomas and W.W. Frye. 1983. Soil properties unchanged after ten years of no-till corn. Agrichemical Age, October-November 276:420-43.
Bock, B.R., and D.E. Kissel. 1988. Ammonia volatilization from urea
fertilizers. Bulletin Y-206.
Bohn, H.L., B.L. McNeal and G.A. O'Connor. 1979. Soil Chemistry.
John Willey & Sons, Inc.
Bray, R.H., and LT. Kurtz. 1945. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 59:39-45.
Bray, Roger H. 1954. A nutrient mobility concept of soil-plant relationships. Soil Sci. 104:9-22.
Bremner, J.M. 1959. Determination of fixed ammonium in soil. J. Agric. Sci. 52:147-160.
Bremner, J.M. 1965. Inorganic forms of nitrogen. In C.A. Black et al. (ed.) Methods of
soil analysis, Part 2. Agronomy 9:1179-1237. ASA and SSSA,
Burford J.R., and J.M. Bremner. 1975. Relationships between the denitrification capacities of soils and total, water-soluble and readily decomposable soil organic matter. Soil Biol. Biochem. 7:389-394.
Cate, R.B., Jr., and
Daigger,
Doran, J.W., and M.S. Smith. 1987. Organic matter management and
utilization of soil and fertilizer nutrients. In J.J. Mortvedt (ed.) Soil
fertility and organic matter as critical components of production systems. Soil
Sci. Soc. Am. SSSA Special Pub. No. 19.
Eberhart
Ernst, J.W., and H.F. Massey. 1960. The effects of several factors on volatilization of ammonia formed from urea in the soil. Soil Sci. Soc. Am. Proc. 24:87-90.
Fenn, L.B., and D.E. Kissel. 1976. The influence of cation exchange capacity and depth of incorporation on ammonia volatilization from ammonium compounds applied to calcareous soils. Soil Sci. Soc. Am. J. 40:394-398.
Finlay, K.W., and G.N. Wilkinson. 1963. The analysis of adaptation in a plant breeding programme. Aust. J. Agric. Res. 14:742‑754.
Follett, R.F. and B. McConkey. 2000. The role of cropland agriculture for C sequestration in the Great Plains. Great Plains Soil Fertility Conference Proceedings.
Fox, R.L., R.A. Olson and H.F. Rhoades. 1964. Evaluating the sulfur status of soils by plant and soil tests. Soil Sci. Soc. Amer. Proc. 28:243-246.
Francis, D.D., J.S. Schepers and M.F. Vigil. 1993. Post-anthesis nitrogen loss from corn. Agron. J. 85:659-663.
Fried, Maurice and L.A. Dean. 1951. A concept concerning the measurement of available soil nutrients. Soil Sci. 73:263-271.
Gillman. G.P. 1979. A proposed method for the measurement of exchange properties of highly weathered soils. Aust. J. Soil Res. 17:129-139.
Graham, E.R. 1959. An explanation of theory and methods of soil
testing.
Harper,
Hauck, R.D., and J.M. Bremner. 1976. Use of tracers for soil and fertilizer nitrogen research. Adv. Agron. 28:219-266.
Hildebrand, Peter E. 1984. Modified stability analysis of farmer managed on-farm trials. Agron. J. 76:271-274.
Kamprath, E.J., W.L. Nelson, and J.W. Fitts. 1956. The effect of pH, sulfate and phosphate concentrations on the adsorption of sulfate by soils. Soil Sci. Soc. Am. Proc. 20:463-466.
Kanampiu, F.K., W.R. Raun and G.V. Johnson. 1997. Effect of nitrogen rate on plant nitrogen loss in winter wheat varieties. J. of Plant Nutr. 20(2&3):389-404.
Kohl, D.H., G.B. Shearer and B. Commoner. 1973. Variation of 15N in corn and soil following applications of fertilizer nitrogen. Soil Sci. Soc. Am. Proc. 37:888-892.
Lindsay, Willard L. 1979. Chemical equilibria in soils. John
Wiley & Sons.
Moll, R.H., E.J. Kamprath and W.A. Jackson. 1982. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron. J. 74:562-564.
Nelson, W.L., A. Mehlich, and E. Winters. 1953. The development, evaluation, and use of soil tests for phosphorus availability. Agronomy 4:153-188.
Olsen, S.R. 1986. The role of ammonium nutrition in higher
yields. p. 6 14. In J. Havlin (ed.) Proc.
Olson, R.V. and C.W. Swallow. 1984. Fate of labeled nitrogen fertilizer applied to winter wheat for five years. Soil Sci. Soc. Am. J. 48:583-586.
Olson, R.A., K.D. Frank, P.H. Grabouski and G.W. Rehm. 1982. Economic and agronomic impacts of varied philosophies of soil testing. Agron. J. 74:492-499.
Olson, R.A., and L.T. Kurtz. 1982. Crop nitrogen requirements,
utilization and fertilization. In Frank J. Stevenson (ed.) Nitrogen in
agricultural soils. Agron. Monogr. 22. ASA, CSSA and SSSA,
Parr, J.F., and R.I. Papendick. 1978. Factors affecting the
decomposition of crop residues by microorganisms. In ______ (ed.) Crop Residue
Management Systems. ASA, CSSA, SSSA.
Pearson, R.W., F. Abruna, and, J. Vicente-
Perry, A.M. 1983. Estimating the greenhouse effect. Science 222:1072.
Polemio, M., and J.D. Rhoades. 1977. Determining cation exchange capacity: A new procedure for calcareous and gypsiferous soils. Soil Sci. Soc. Am. Proc. 32:488-492.
Ranney, R.W. 1969. An organic carbon-organic
matter conversion equation for
Raun, W. R., H. J. Barreto, and R. L. Westerman. 1993. Use of stability analysis for long-term soil fertility experiments. Agron. J. 85:159-167.
Shearer, Georgia, and J.O. Legg. 1975. Variations in the natural abundance of 15N of wheat plants in relation to fertilizer nitrogen applications. Soil Sci. Soc. Amer. Proc. 39:896-901.
Thomas, Grant W. 1982. Exchangeable cations. In A.L. Page, R.H. Miller and D.R. Keeney (eds.) Methods of soil
analysis. Part 2, 2nd ed. Agron. Monogr. 9. ASA and SSSA,
Tisdale, Samuel L., Werner L. Nelson, James D. Beaton and John L.
Havlin. 1993. Soil fertility and fertilizers. Fifth Edition. MacMillan
Publishing Co.
Tinoco, Ignacio, Kenneth Sauer and James C. Wang. 1978. Physical
chemistry, principles and applications in biological sciences. Prentice-Hall,
Inc.
Varvel, G.E., and Todd Andrews Peterson. 1991. Nitrogen fertilizer recovery by grain sorghum in monoculture and rotation systems. Agron. J. 83:617-622.
Vigil, M.F., and D.E. Kissel. 1991. Equations for estimating the amount of nitrogen mineralized from crop residues. Soil Sci. Soc. Am. J. 55:757-761.
Vose, P.B. Introduction to nuclear techniques in agronomy and
plant biology. 1980. Pergammon Press Inc.,
Wallace, Arthur, Garn A. Wallace and Jong W. Cha. 1990. Soil organic matter and the global carbon cycle. J. of Plant Nutr. 13:459-466.
Wittwer, S.H. 1985. Carbon dioxide levels in the biosphere: effects on plant productivity. CRC Critical Reviews in Plant Sciences 2:171-198.
Wuest, S.B., and K.G. Cassman. 1992. Fertilizer-nitrogen use efficiency of irrigated wheat: I. Uptake efficiency of preplant versus late-season application. Agron. J. 84:682-688.
Yates F, and W.G. Cochran. 1938.
The analysis of a group of experiments. J. Agric. Sci. 28:556‑580.
1000000 = 106 = mega
 1000
= 103 = kilo
1000
= 103 = kilo
100 = 102 = hecto
10 = 101 = deka
0.1 = 10-1 = deci
0.01 = 10-2 = centi
0.001 = 10-3 = milli
0.000001 = 10-6 = micro
0.000000001 = 10-9 = nano
Appendix Table
1. Conversion factors and relationships between English and metric units.
______________________________________________________________________
Yield and
Rate
lb/ac
* 1.12 = kg/ha
bu/ac
* 67.2 = kg/ha (60 lb test weight)
bu/ac
* 0.0672 = Mg/ha (60 lb test weight)
1
Mg/ha = 14.88 bu/ac (60 lb test weight)
Area
1
hectare = 10000 m2
1 acre
= 43560 ft2
1 acre
(ac) = 0.405 hectares (ha) 1
ha = 2.47 ac
Length
1 inch
(in) = 2.54 centimeters (cm) 1
cm = 0.393 in
1 foot
(ft) = 30.48 centimeters (cm)
1 mile
(mi) = 1.609 kilometers (km); 1 mile=5280ft 1
km = 0.621 mi
1 yard
(yd) = 0.914 meters (m) 1
m = 1.094 yd
1 mile2 (mi) = 259 hectares (ha)
Volume
1
gallon (gal) = 3.785 liters (l) 1
l = 0.264 gal
1
quart (qt) = 1.057 liters (l) 1
l = 0.964 qt
Mass
1
kilogram (kg) = 1000 grams (g)
1
Megagram (Mg) = 1000 kilograms (kg)
1
ounce (oz) = 28.35 grams (g) 1
g = 0.03527 oz
1
pound (lb) = 0.454 kilograms (kg) 1
kg = 2.205 lb
1 ton
(2000 lb) = 907 kilograms (kg)
Temperature
Centrigrade
(°C) = 5/9 (°F - 32)
Fahrenheit
(°F) = (9/5 °C) + 32
______________________________________________________________________


![]()
Form taken up by plant: NH4+, NO3-
Mobility in soil: NH4+: no; NO3-: yes
NO3- water soluble, not influenced by soil colloids
Mobility in plant: Yes
Deficiency
symptoms: Chlorosis in older leaves, under severe deficiency
lower leaves are brown, beginning at the leaf tip and proceeding along the
midrib.
Soil
pH where deficiency will occur: None due to nitrate's mobility
Role of nutrient in plant growth: N assimilation into amino acids for protein and amino acid synthesis, component of chlorophyll, vegetative growth
Enzymes that require N: Nitrate reductase, nitrite reductase, nitrogenase
Role of nutrient in microbial growth: Necessary for the synthesis of amino acids
Concentration in plants: Wheat 1.7 - 3.0%
Grain 2.0%
Forage 3.0 %
Straw
Corn 2.7 - 3.5%
Soybeans 4.2 - 5.5%
Grain sorghum 3.3 - 4.0%
Peanuts 3.5 - 4.5%
Alfalfa 4.5 - 5.0%
Bermudagrass 2.5 - 3.0%
Effect of pH on availability:
Precipitated forms (low pH): none
Precipitated forms (high pH): none
at pH>8, no nitrification; at pH>7, NO2- accumulates
Interactions with other nutrients: Si: enhances leaf
erectness, thus neutralizing the negative effects of high nitrogen supply on
light interception (leaf erectness usually decreases with increasing nitrogen
supply); P: symbiotic legume fixation needs adequate P or a N deficiency can
result; Mo: component of nitrogenase therefore could have Mo induced N
deficiency in N2 fixing legumes (especially under acid soils
conditions); Fe: necessary for nitrogenase and ferredoxin (electron carrier),
legume hemoglobin, deficiency reduces nodule mass, and nitrogenase;
Fertilizer sources: ammonium
sulfate, anhydrous ammonia, ammonium chloride, ammonium nitrate, ammonium
nitrate-sulfate, ammonium nitrate with lime, ammoniated ordinary
superphosphate, monoammonium phosphate, diammonium phosphate, ammonium
phosphate-sulfate, ammonium polyphosphate solution, ammonium thiophosphate
solution, calcium nitrate, potassium nitrate, sodium nitrate, urea,
urea-sulfate, urea-ammonium nitrate, urea-ammonium phosphate, urea phosphate.
References:
Burford, J.R., and J.M. Bremner. 1975. Relationships between the denitrification capacities of soils and total, water-soluble and readily decomposable soil organic matter. Soil Biochem. 7:389-394.
Marschner, Horst.
1995. Mineral Nutrition in Higher
Plants. Academic Press,
Tisdale, S.L., W.L. Nelson, J.D. Beaton, and
J.L. Havlin. 1993. Soil Fertility and Fertilizers. MacMillan Publishing Co.,
Authors:
Heather Lees, Shannon Taylor, Joanne LaRuffa and Wade Thomason