| Average | 88.45 | |
| Median | 88 | |
| Std_Dev | 5.53 |
| Student Questions | Avg. | ||||||||||||
| 1 | 1 | 0 | 2 | 3 | 3 | 0 | 0 | 1 | 0 | 1 | 0 | 1.00 | |
| 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0.36 | |
| 3 | 1 | 1.5 | 2.5 | 0.5 | 0 | 0.5 | 0.5 | 0.5 | 1.5 | 1 | 0 | 0.86 | |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0.05 | |
| 6 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 2 | 0.45 | |
| 7 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0.27 | |
| 8 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.36 | |
| 9 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.09 | |
| 10 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0.18 | |
| 11 | 3 | 0 | 2 | 2 | 4 | 0 | 2 | 0 | 1 | 3 | 1 | 1.64 | |
SOIL5813
Soil-Plant Nutrient Cycling and Environmental Quality
First
Hour Exam
February
8, 2002
Name:
______________
Miscellaneous:
T
F
An example of protonation is when NH3 reacts with H+
to become stable NH4
T F
The adverse effects of weather can be overcome by applying more
N
T
F
H+ does not have an electron, and that is why it has
a + charge, having only 1 proton
T
F
Subsoils can provide significant amounts of NH4 for crop
growth
T
F
NH4 is most likely to accumulate in semi-arid
environments
T
F
Nitrogen use efficiency for worldwide cereal production averages
67%
T
F
Work by Francis et al (1993) reported that plant N losses accounted
for 52 to 73% of the unaccounted-for N in 15N balance
calculations
T
F
Denitrification in soils is controlled largely by the supply of
water-soluble or readily decomposable organic matter
T
F
Denitrification losses are generally greater when N is applied in
conventional till soils as compared to zero till soils.
T
F
The vast majority of NOx is released as nitric oxide,
NO, which converts to nitrogen dioxide, NO2, within minutes by
reaction with ozone and peroxy radicals.
NO2 is recycled to NO by photolysis.
STUDENT Questions
1.
Under what climatic conditions does soda niter (sodium nitrate) form? In
other words, what climatic conditions would lead to the accumulation of
nitrate in the subsoil? (Jamie
Patton)
Soda
niter is an evaporite that forms in arid to semi-arid climates. We
can
hypothesize that in these environments during times of moisture,
mineralization
and nitrification take place more rapidly than during
periods
of low moisture, as microbes, like almost all life, are dependent
on
water to survive and carry out their life processes.
Because the
precipitation
events in these environments are infrequent and often
torrential,
there is the potential for leaching of nitrate out of the root
zone.
However, because of the extreme desiccation of the soil one would
not
expect the wetting front to move to great depths, resulting in the
accumulation
of nitrate at the leading edge of the wetting front (cm to m
in the
soil). Over a
period of hundreds to thousands of years nitrate
would
accumulate at semi-shallow depths and react with the base cations in
the
soil to form soluble nitrate salts such as soda niter.
2.
Compare NH4 in arid/semi-arid environments vs. humid
environments. Include
formation, transformations, and fates (Randy Davis)
Humid:
As organic matter is accumulated at the soil surface,
mineralization
is
rapid due to favorable environmental conditions.
As quickly as NH4 is
produced
it is immediately taken up by plants leaving little or none to
undergo
nitrification.
Arid/Semi:
As water is limiting in this type of environment smaller
populations
of plant life are present. Consequently
there is little organic
matter
accumulation. However, as N
in residue is mineralized it also
undergoes
nitification. As water is the
limiting nutrient it is also
important
that other plant nutrients, i.e. NO3, be transported by water. NH4
may
also accumulate leading to possible loss as NH3.
3.
What are the effects of soil pH on nitrification? (Yan Tang)
Nitrifying
organisms are sensitive to H+.
In
pure culture, their activity is reduced below pH 6.0 and becomes
negligible
below pH 5.0.
Some
soils with pH 4.0 or less, however, may contain some NO3- and it
appears
that the organisms derived from acid soils are frequently more
tolerant
of H+.
Optimum
pH is 6.6 to 8.0 or higher.
4.
Name 3 different pathways by which N is lost from ecosystems and
describe
the environmental conditions in which they are apt to happen? (Jason
Lawles)
a)
Ammonia volatilization happens when there is a high pH generally
above
7.5 and drying weather conditions in semi-arid and arid climates
b)
Leaching occurs when NO3- is moved by water below
the root zone.
Leaching
occurs in environments where there is ample rainfall, soil type
also
influences how rapid NO3- moves through the soil.
c)
Denitrification. When the soil is very wet, water fills in the spaces
between
soil particles. This leaves very little room for oxygen. Some
soil
microorganisms can get the oxygen they need from the oxygen portion
of the
nitrite (NO2-) and nitrate (NO3-)
forms of nitrogen. When this
happens,
nitrogen (N2) and nitrous oxide (N2O) gas are formed
and escape
to the
atmosphere.
5.
What happens to NH4+ and NO3-
in soil? (Jitao)
1.About
NH4+:
-
Immobilization.
When residue does not contain enough N to meet the needs of microbes
decaying it, the microbes will utilize NH4+ in
the residue and any additional mineral-n present in the soil. This
process is called immobilization.
-
Cation
exchange. When NH4+ accumulated in the soil, NH4+
will successfully compete for exchange sites on clay and humus
occupied by other cation. Then NH4+ becomes
immobile in the soil.
-
Volatilization.
When the pH is high, the N could be loss by volatilization of the gas
NH3.
NH4+
+ OH- <-->
NH3 + H2O?
Surface
drying and removing H2O shift the equilibrium in favor of the
reaction to the right.
-
Plant
uptake. Plants can uptake NH4+ directly. As NH4+
is immobile in the soil, the density of the root is very important for
the plant uptake.
-
Nitrification.
NH4+ change to NO3-. This
process is called nitrification.
2NH4+
+4O2 ßŕ
2NO3- + 4H+ + 2 H2O
1.About
NO3-:
-
Immobilization.
When residue does not contain enough N to meet the needs of microbes
decaying it, the microbes will utilize NO3- in
the residue and any additional mineral-n present in the soil. This
process is called immobilization.
6.
Some states do not recommend soil testing for inorganic nitrogen for all
general purposes. Give a
reason why you think they do not. Do
you think they should soil test for inorganic nitrogen?
If so, why? (Roger Teal)
I
have designed this question to be opinionated because I don’t think that
everyone realizes that nitrogen analysis is not always a given in soil
testing.
Although
there is no wrong answer to this question, the rationale behind not
testing for nitrogen without a request is: inorganic nitrogen soil test
would not be accurate. In
these regions, the soil temperature stays above 50oF most of
the time, the soils are acid with no more than 1% OM and low CEC (kaolinite),
and the annual rainfall is above 50 inches.
In these conditions the inorganic nitrogen levels are consistently
changing with high potential for leaching and immobilization
7.
A farmer applied excessive rates of nitrogen fertilizer with the intention
to
increase yield. He applied the fertilizer at planting when the moisture
and
temperture was optimal. Describe two problems arising from these
excessive
rates in the soil system (Kefyalew Girma)
a.
Excess nitrogen as NO3-N is fairly easy to leach from
the
soil.
The consequence of this is nitrogen level build up in ground
water
that is then used by animals or humans.
b.
Nitrification of the added fertilizer can lower the pH of the
soil.
This in turn may enhance Al and/or Mn toxicity.
8.
In what way does nitrogen cycle pose problem to environment?
(Adi Malapati)
If
excess amounts of N in the form of nitrate and ammonium are present on the
surface of soils, these get washed away as surface runoff in to the nearby
water bodies. Nitrogen
entering into the water bodies can cause’ eutropication’.
Ammonium is not only toxic to fish but it also uses up the
dissolved oxygen in the water during the nitrification process.
N
in the form of nitrate poses a particular environmental concern because of
its leaching properties. Nitrate
can reach the ground water through leaching and can create health problems
if its content is more than 10 ppm
In saturated soils or the wet lands where rice is grown under water logged
conditions denitrification is the major process because anerobic
conditions prevail. Nitrous
and nitric oxides are the product
of this process and both these are greenhouse gases.
9.
You have a paper factory in a small country where you don't have
big disposal sites and you have a lot of waste paper and sawdust from a
paper factory that you have to dispose of. One of your subordinates told
you that paper is organic in nature, and wanted to incorporate it in the
soil in adjacent wheat and corn fields. Is this a good idea?
Yes or No? Why? (Jagadeesh)
I
think it would not be a good idea because if you incorporate a lot of
paper it will
tend
to immobilize the nitrogen as the C:N ratio would be very high. Plants would likely be highly stressed due to the limited
supply of N.
10.
Explain Ammonia (NH3) volatilization in terms of pH
(generally) by means of a
graph or a chemical equation with an explanation for whichever you choose.
For example
under what conditions will ammonia be volatilized? (Micah Humphreys)
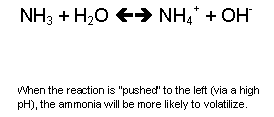
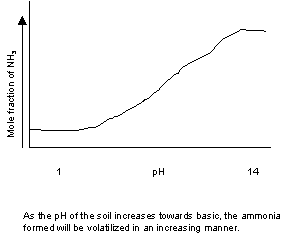
11.
What does inorganic nitrogen buffering mean?
Define 4 of the buffering mechanisms? (Shambel Moges)
Inorganic
nitrogen buffering is the ability of the soil-plant system to control the
amount of inorganic N accumulation in the rooting profile when N
fertilization rates exceed that required for maximum yield.
Some
of the important mechanisms include:
1.
increased applied N results in increased plant N loss (NH3)
2.
higher rate of applied N results in increased volatilization losses
3.
higher rate of applied N can result in increased denitrificaiton
losses
4.
application of higher N rates will increase grain protein, forage
and straw N.
Miscellaneous
(from lecture material)
1.
The application of excessive N rates in cereal production has been
blamed for
a.
‘dead zone’ or hypoxia in the Gulf of Mexico
b.
eutrophication of lakes and streams
c.
nitrate contamination of groundwater
d.
global warming via increased production of nitrous oxide from
denitrification
e.
negative backlash of the green revolution
2.
What is the relationship between greenhouse warming and
denitrification in soils? Explain?
-
Increasing
use of N fertilizers has sent more Nitrous oxide into the atmosphere
via denitrification (nitrous oxide generated by bacteria using
nitrate)
-
Reactions
of nitrous oxide with excited oxygen contribute to the destruction of
ozone
-
Ozone
molecules serve to screen out dangerous ultraviolet light, and nitrous
oxide (from denitrification) can destroy ozone
-
The
atmospheric lifetime of nitrous oxide is longer than a century, and
every one of its molecules absorbs roughly 200 times more outgoing
radiation than does a single carbon dioxide molecule.
3.
What concentrations of soil NH4, NO2, and NO3
(mg/kg) would you expect to find in an undisturbed, native prairie
environment? Explain.
5 ppm
NH4
5 ppm
NO3
<1
ppm NO2
none
of these are expected to accumulate in a native prairie soil.
4.
What concentrations of NH4 (in soil) would you expect to
be toxic?
NH4
itself would not be toxic, excluding the salt effect at high rates.
What would be toxic would be NH3
5.
What are likely the three most important factors controlling what
actually happens in the nitrogen cycle?
a.
soil organic matter, temperature, and pH
b.
soil organic matter, moisture, and pH
c.
soil organic matter, pH, and temperature
d.
soil organic matter, temperature, and moisture
e.
soil organic matter, pH, and, volatilization
6. Burford and Bremner found the following
a.
denitrification capacities were highly correlated with water
soluble carbon
b.
denitrification capacities were highly correlated with
mineralizable carbon
c.
denitrification under anaerobic conditions in controlled largely by
the supply of readily decomposable organic matter
d.
denitrifying bacteria responsible for reduction of nitrate to
gaseous forms of nitrogen are facultative anaerobes that have the ability
to use both oxygen and nitrate as hydrogen acceptors
BONUS:(5
points)
Outline
the countries of Colombia, Peru, Pakistan, Nepal, and France